- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Penetration testing training course description An advanced technical hands on course focusing on hacking and counter hacking. The course revolves around a series of exercises based on "hacking" into a network (pen testing the network) and then defending against the hacks. What will you learn Perform penetration tests. Explain the technical workings of various penetration tests. Produce reports on results of penetration tests. Defend against hackers. Penetration testing training course details Who will benefit: Technical support staff, auditors and security professionals. Staff who are responsible for network infrastructure integrity. Prerequisites: IP Security IP VPNs Duration 5 days Penetration testing training course contents Introduction Hacking concepts, phases, types of attacks, 'White hacking', What is penetration testing? Why use pen testing, black box vs. white box testing, equipment and tools, security lifecycles, counter hacking, pen testing reports, methodologies, legal issues. Physical security and social engineering Testing access controls, perimeter reviews, location reviews, alarm response testing. Request testing, guided suggestions, trust testing. Social engineering concepts, techniques, counter measures, Identity theft, Impersonation on social media, Footprints through social engineering Reconnaissance (discovery) Footprinting methodologies, concepts, threats and countermeasures, WHOIS footprinting, Gaining contacts and addresses, DNS queries, NIC queries, ICMP ping sweeping, system and server trails from the target network, information leaks, competitive intelligence. Scanning pen testing. Gaining access Getting past passwords, password grinding, spoofed tokens, replays, remaining anonymous. Scanning (enumeration) Gaining OS info, platform info, open port info, application info. Routes used, proxies, firewalking, Port scanning, stealth port scanning, vulnerability scanning, FIN scanning, Xmas tree scanning, Null scanning, spoofed scanning, Scanning beyond IDS. Enumeration concepts, counter measures and enumeration pen testing. Hacking Hacking webservers, web applications, Wireless networks and mobile platforms. Concepts, threats, methodology, hacking tools and countermeasures. Trojan, Backdoors, Sniffers, Viruses and Worms Detection, concepts, countermeasures, Pen testing Trojans, backdoors, sniffers and viruses. MAC attacks, DHCP attacks, ARP poisoning, DNS poisoning Anti-Trojan software, Malware analysis Sniffing tools. Exploiting (testing) vulnerabilities Buffer overflows,, simple exploits, brute force methods, UNIX based, Windows based, specific application vulnerabilities. DoS/DDoS Concepts, techniques, attack tools, Botnet, countermeasures, protection tools, DoS attack pen testing. SQL Injection Types and testing, Blind SQL Injection, Injection tools, evasion and countermeasures. Securing networks 'Hurdles', firewalls, DMZ, stopping port scans, IDS, Honeypots, Router testing, firewall testing, IDS testing, Buffer Overflow. Cryptography PKI, Encryption algorithms, tools, Email and Disk Encryption. Information security Document grinding, privacy.

A System Approach to Good Pharmacovigilance Practice
By Research Quality Association
Course Information In today's evolving landscape, pharmacovigilance (PV) systems face ongoing challenges due to global, national, and company-specific events. This course focuses on developing personnel equipped to navigate these complexities and improve the safe use of medicinal products. We emphasise continuous global thinking, communication, and strategic planning, ensuring adaptability across various levels. The course explores maintaining PV system functionality while adhering to regulatory requirements. Participants will apply European regulatory standards to enhance and sustain PV system effectiveness, contributing to improvement initiatives and ensuring operational integrity. The course covers PV system intricacies, regulatory compliance, and a 'systems approach' for auditors, quality assurance personnel, and PV practitioners. Through presentations and workshops, attendees gain insights into implementing and maintaining an effective PV system. Who should attend? Auditors Pharmacovigilance Quality System Managers Pharmacovigilance scientists The QPPV. Course benefits Throughout the course delegates will explore application of the legal requirements for the PV system and quality system and how to assure these systems. They will demonstrate their ability to contribute to: A systematic investigation of the pharmacovigilance system and its quality system Examination of how the pharmacovigilance system and quality system interact to achieve compliance. The risk-based approach to auditing the PV system and quality system The maintenance of 'inspection readiness' Explore how to investigate the complex PV system Discussions about how to monitor and maintain the PV system and assure compliance. Course Objectives Clarify what has to be done: Explore application of the legal requirements. Explore how to do what has to be done: Adopt a systemic approach to systematically investigate or implement and maintain the PV system and quality system Examine how a compliant PV system and a compliant quality system interact to achieve compliance with regulatory requirements for PV Explore how to investigate the complexity of the PV system. Discus how to identify what is missing or what needs to be improved: Discuss how to monitor and maintain the PV system and assure compliance. This course will assist delegates with: An understanding of key system principles, A practical approach to implementing, maintaining and monitoring the PV system and its quality system A procedure to share expertise to increase efficiency and confidence. This course is structured to encourage delegates to: Discuss and develop ideas - Share knowledge and experiences - Solve specific problems. By the end of the course delegates will be able to: Understand better the pharmacovigilance system, its quality system and how the components interact to achieve the objectives of pharmacovigilance Investigate, and analyse the pharmacovigilance system and to identify what is missing and what needs to be improved. Tutors Tutors will be comprised of (click the photos for biographies): Jana Hyankova Head of PV Department, IVIGEE Services a.s. Programme Please note timings may be subject to alteration. Day 1 08:30 Welcome, registration, course objectives and introduction to work groups Housekeeping notices, meet other delegates, explore how to work in your work group, course objectives. Clarify the definition and objectives of Pharmacovigilance. 09:30 The Regulatory Framework for Pharmacovigilance Identify the relevant regulations and directives. Explore GVP guidance, structure of the modules and standard format of each module. 10:00 The Pharmacovigilance System Exploration of how to organise what has to be done, communications. Exploration of the structures and processes for pharmacovigilance. 10:30 Break 11:00 Workshop 1 and Feedback Exploring an organisational model of the pharmaceutical company- cooperation between PV and other stakeholders. 12:00 The Quality System for pharmacovigilance Exploration of the structures, processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 13:00 Lunch 14:00 Workshop 2 and Feedback The quality system puzzle. Explore the organisation of the PV quality system and how it interacts with the PV system. 14:30 The Quality System for pharmacovigilance Exploration of the structures processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 15:00 Description of PV System 15:30 Break 15:30 Workshop 3 and Feedback The quality system puzzle Explore the organisation of the PV quality system and how it interacts with the PV system. 16:00 The Pharmacovigilance Safety Master File Construction of the Pharmacovigilance System Master File and its purpose. 17:00 Workshop 3 and Feedback Description of PV System. 18:00 End of Day Day 2 08:30 Drug Safety in the Clinical Trial Environment - Part 1 Information flow and responsibilities of the sponsor. 09:30 Workshop 4 and Feedback Drug Safety in the Clinical Trial environment: Information flow and responsibilities of the sponsor. 10:30 Break 11:00 Drug Safety in the Clinical Trial Environment - Part 2 Information flow and responsibilities of the sponsor. 12:00 Lunch 13:00 Workshop 5 and Feedback Drug Safety in the clinical trial environment: Information flow and responsibilities of the sponsor. 13:30 Processing of Safety Data Exploration of safety data processing, verification, validation, follow up, formatting and collation, reporting requirements, quality and data management. 15:00 Break 15:30 EudraVigilance Exploration of how EudraVigilance supports the PV system. 16:15 Signal Detection and Evaluation/Risk Benefit Assessment: Pharmacovigilance Risk Assessment Committee (PRAC): What is a signal? What are the regulatory requirements? How is signal detection and evaluation conducted? Qualitative and quantitative methods of signal detection. Risk benefit assessment. 17:00 Risk Management Plans A cornerstone of Pharmacovigilance safety communications, direct healthcare professional communication 18:00 End of Day Day 3 08:30 The Pharmacovigilance Risk Assessment Committee (PRAC) Exploration of how good practice is achieved. Composition, role and responsibilities. Examples of referrals. 09:15 Development Safety Update Reports (DSURs): Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:30 Break 11:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 12:00 Workshop 6 and Feedback To explore the compilation and submission of the PSUR. 13:00 Lunch 13:30 Role of the QPPV Exploration of the legal responsibilities of the QPPV and the MAH. 14:30 Break 15:00 Workshop 7 and Feedback To explore the challenges faced by the QPPV. 15:30 End of course Extra Information Face-to-Face Course Course material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device< Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD Points 23 Points Development Level Develop

Assessing and Managing Cyber Security Risks for Essential Services Operators
By EnergyEdge - Training for a Sustainable Energy Future
About this Virtual Instructor Led Training (VILT) This 3 half-day Virtual Instructor Led Training (VILT) course will help participants grasp the idea of real-world risk management and how this relates to the cyber world. The VILT course will cover topics surrounding identifying cyber risks and vulnerabilities, guidance on applying administrative actions, and comprehensive solutions to ensure your organization is adequately secure and protected. The VILT course will guide participants on how to conduct a security risk assessment for their organization, and equip them with the skills to develop a risk compliance assessment plan as well as methods to develop risk management strategies which can improve their organization's security posture. The VILT course has at least a 30% hands-on approach through the use of Table Top Exercises. The VILT course will cover the following modules: Introduction to Risk Assessments (RA) Threat Actors and Their Motivations Threat and Risk Assessment Critical Controls Identification Maturity Assessment Treated Cyber Risk Profile Target Cyber Risk Profile and Strategy Target Audience The VILT course is intended for professionals responsible for organizational information and security system and those involved in operating and maintenance of critical information and IT network & sotware systems. Professionals who are designated as the Single Point of Accountability (SPoA) as well as system auditors will find this course useful. Course Level Basic or Foundation Training Methods The VILT course will be delivered online in 3 half-day sessions comprising 4 hours per day, with 2 x 10 minutes break per day, including time for lectures, discussion, quizzes and short classroom exercises. Course Duration: 3 half-day sessions, 4 hours per session (12 hours in total). This VILT course is delivered in partnership with ENGIE Laborelec. Trainer Your expert course leader is a is specialized in cybersecurity risk management. Before joining ENGIE, she worked for The National Cybersecurity Agency of France (ANSSI) based in Paris (France) and for Deloitte Belgium located in Zaventem (Belgium). She has been involved in cybersecurity projects focusing on the principle of protecting critical infrastructures. Her different experiences in Cyber Security, Anti-Money Laundering and Global Trade Compliance (including Export Control and Customs) gave her the opportunity to use methodologies tackling strategic, operational and financial control issues at all levels of an organization: people, business processes, IT applications and infrastructure, legal and regulatory compliance. She was an EBIOS Risk Manager (RM) trainer while she worked for the French government; EBIOS RM is the French method for assessing and treating digital risks. She also had the opportunity to represent France towards European institutions and other relevant stakeholders for topics related to cybersecurity risk management. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information about post training coaching support and fees applicable for this. Accreditions And Affliations

IFRS Accounting for the Oil and Gas Sector
By EnergyEdge - Training for a Sustainable Energy Future
Gain expertise in IFRS accounting for the oil and gas sector with our industry-focused training course. Enroll today with EnergyEdge.

IP security training course description Connection to the Internet is becoming an essential business tool. This course looks at firewalls, digital certificates, encryption and other essential topics for e-commerce sites. A generic course that looks at firewalls and VPNs. Hands on sessions include using hacking tools and configuring firewalls. What will you learn Describe: Basic security attacks RADIUS SSL IPSec VPNs Implement digital certificates Deploy firewalls to protect Web servers and users. Secure Web servers and clients. IP security training course details Who will benefit: Network administrators. Network operators. Security auditors Prerequisites: TCP/IP foundation for engineers Duration 2 days IP security training course contents TCP/IP review Brief overview of the relevant headers. Hands on Download software for course, use analyser to capture passwords on the wire. Security review Policies, Types of security breach, denial of service, data manipulation, data theft, data destruction, security checklists, incident response. Security exploits The Internet worm, IP spoofing, SYN attack, hijacking, Ping o' Death⦠keeping up to date with new threats. Hands on Use a port scanning tool, use a 'hacking' tool. Firewalls Products, Packet filtering, DMZ, content filtering, stateful packet inspection, Proxies, firewall architectures, Intrusion Detection Systems, Viruses. Hands on Set up a firewall and prevent attacks. NAT NAT and PAT, Why use NAT, NAT-ALG, RSIP. Encryption Encryption keys, Encryption strengths, Secret key vs Public key, algorithms, systems, SSL, SSH, Public Key Infrastructures. Hands on Run a password-cracking program. Authentication Types of authentication, Securid, Biometrics, PGP, Digital certificates, X.509 v3, Certificate authorities, CRLs, PPP authentication, RADIUS. Hands on Using certificates. Web client and server security Cookies, browser certificates, censorship, PICS. Operating system security, Web server user authentication, Restricting access, Logging, Securing CGI scripts. Hands on Browser security. VPNs and IPSec What is a VPN, tunnelling, L2F, PPTP, L2TP, IPSec, AH, ESP, transport mode, tunnel mode.

Tripod Beta Practitioner - Bronze Level
By EnergyEdge - Training for a Sustainable Energy Future
About this VILT Tripod can be used in any area of business where the organisation has a management system in place to prevent unwanted events e.g., health, safety, environment, quality, security, productivity, project management, and many more Tripod Beta is one of several tools based on Tripod's fundamental principles. Tripod Beta is based on proven theories, man years of academic research, and testing in the workplace. The Swiss Cheese Model originated from this work. Features of the methodology are: 1) the Tripod Beta diagram; it provides an easy-to-read summary of the entire investigation on a single page, 2) it accommodates deficiencies in leadership and worker participation, 3) it accommodates deficiencies in human behaviour, and 4) it highlights missing controls (not just controls that failed). Quality throughout all aspects of Tripod is assured by the Stichting Tripod Foundation (STF) and the Energy Institute. The participants will gain a theoretical understanding of the Tripod Beta methodology and terminology. They will be able to read Tripod diagrams and reports, and be able to assist incident investigation/analysis as a team member. This is the first step to becoming an accredited silver or gold practitioner. Tripod Beta Practitioner Accreditation is meant to build these skills, through a blend of support, coaching and assessments. Feedback is provided on Tripod incident investigation reports, giving the Practitioner the opportunity to hone their skills and become confident in their ability to use Tripod effectively. Training Objectives Upon completion of this course, participants will be able to: Pass the Tripod Beta Practitioner (Bronze Level) exam Act as a team member or Tripod facilitator on an incident investigation Plan and schedule activities for an incident investigation Focus line of enquiry during an investigation Engage with the most relevant people at each stage of the investigation Describe the incident causation paths in terms that align with their management system Consider issues relating to leadership, worker participation and human behaviour Assess the quality of an incident report Apply the process to any type of unwanted event that should have been prevented by a management system e.g., health, safety, environment, financial, security, productivity, quality, project management etc. Combine the findings from many incidents with data from other initiatives e.g., audits and inspections, to spot trends to prioritise actions and product a single improvement plan Target Audience The course is recommended for anyone who is expected to play a role in designing, reviewing, auditing, and following your organizations OH&S management system. Successful participants will be awarded the Stitching Tripod Foundation Tripod Beta Bronze certificate. The following oil & gas company personnel will benefit from the knowledge shared in this course: CEO Team Leaders Legal, insurance and finance departments Managers (Line and Function) Maintenance Engineers Quality Assurance Engineers Process Engineers Incident Investigators (Team member & Tripod facilitator) Project Managers System Custodians Technical Authorities Key Contractor's Management Contract Managers/Holders Safety Representatives Risk Management Engineers HSE Advisors Supervisors Auditors Regulators Course Level Basic or Foundation Trainer Your expert course leader has over 30 years of experience in construction, operations and maintenance with the upstream exploration and production sector. He joined Shell International E&P in 1971 and for 28 years worked in several locations around the world. Following the Piper Alpha incident he led Shell's two year, £10M major overhaul of their permit to work system. He first made use of the Tripod Beta principles during this period and since then he has delivered over 100 Tripod Beta courses in more than 25 locations around the world. He is a Chartered Engineer, a member of the Institution of Engineering and Technology and holds a postgraduate diploma from the University of Birmingham (UK). POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information post training support and fees applicable Accreditions And Affliations

Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

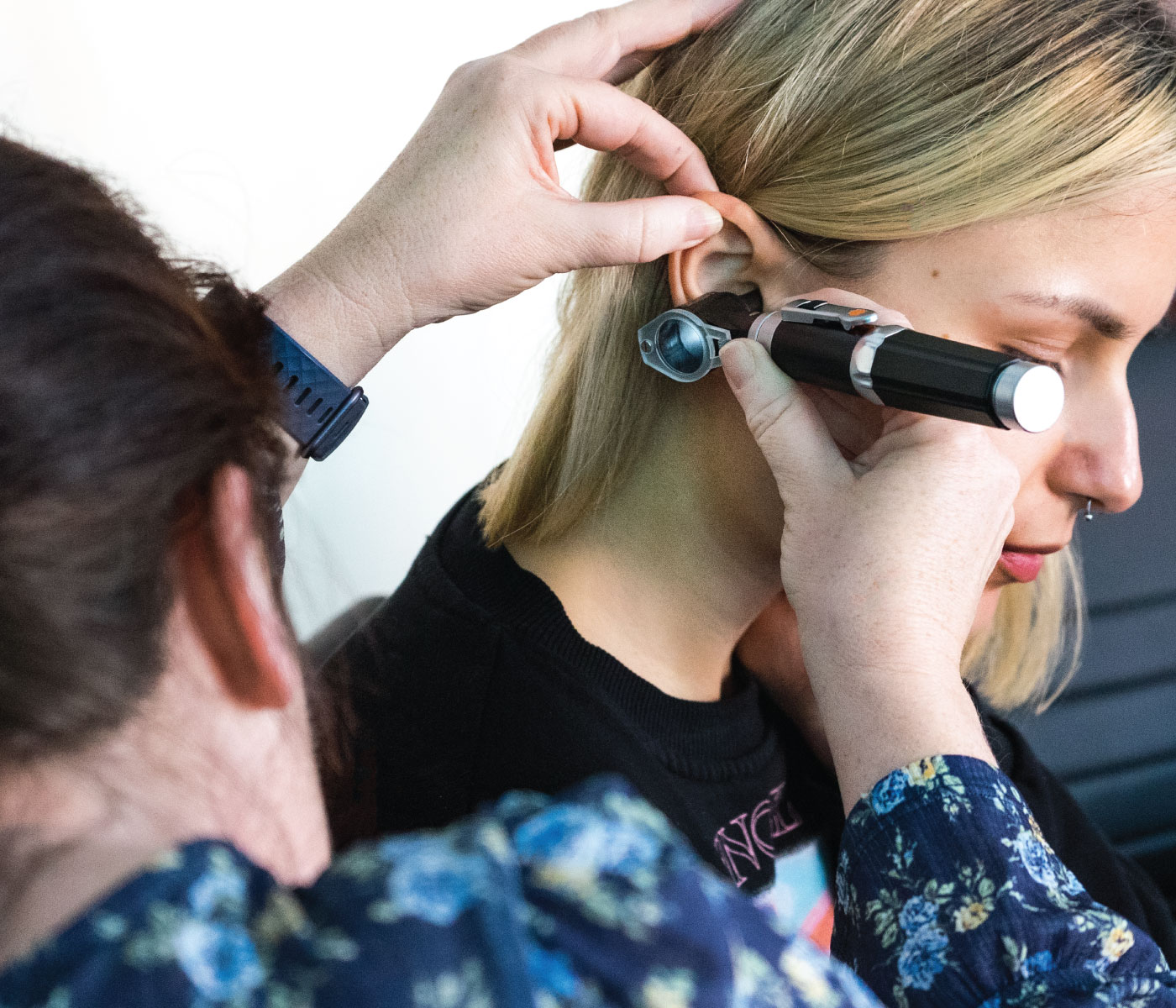
An Understanding of Ear Assessment and Care Update (including Ear Irrigation)
By Guardian Angels Training
Enhance your ear assessment and care skills with our comprehensive course on ear irrigation. Gain practical knowledge and updated insights for optimal ear hygiene.
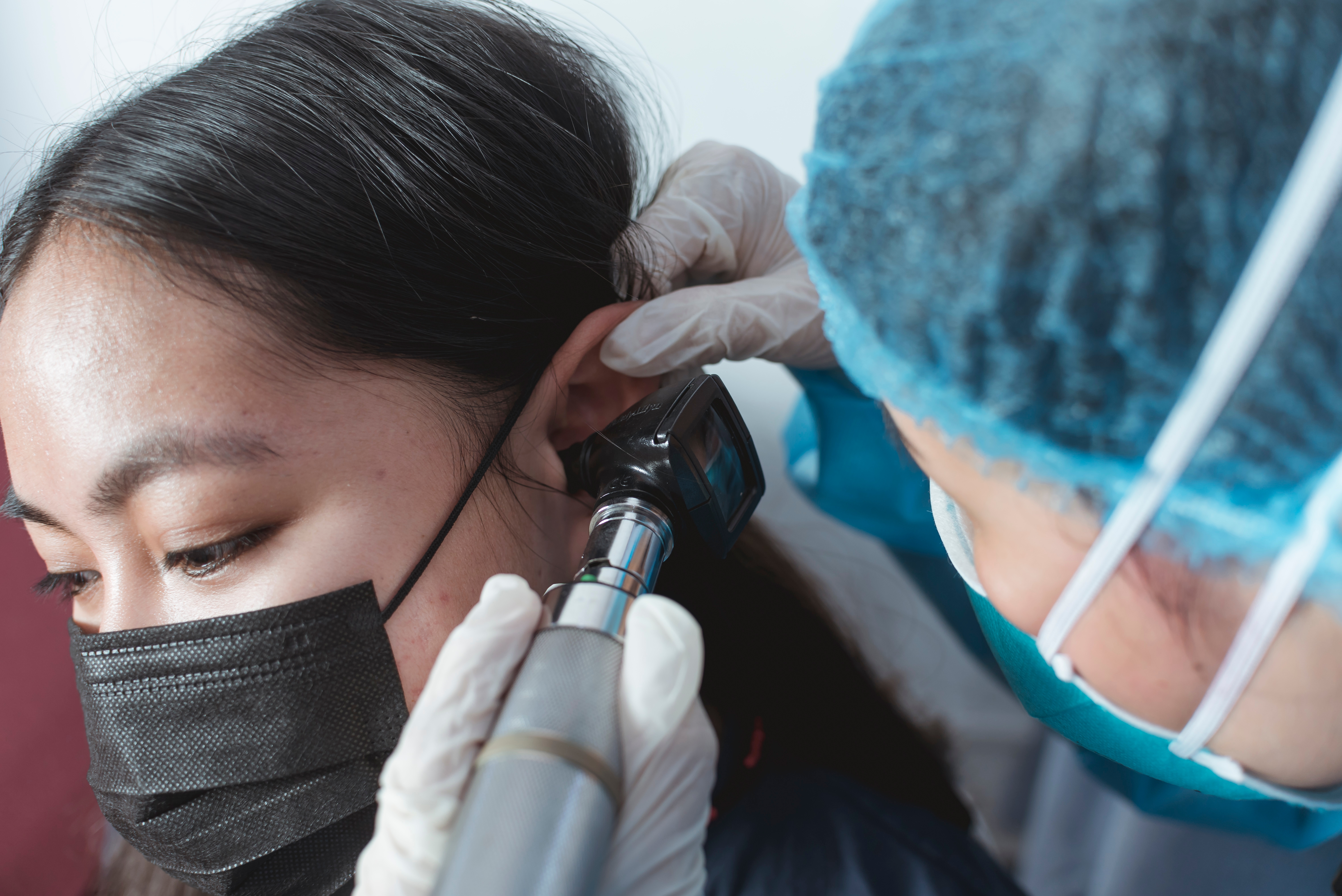
An Understanding of Ear Anatomy and Otoscopy
By Guardian Angels Training
Gain comprehensive knowledge of ear anatomy and otoscopy with our course. Ideal for healthcare professionals, medical students, and audiologists.
Overview Internal auditing is an independent and objective activity to evaluate an organisation's internal operations. You'll learn how to initiate an audit, prepare and conduct audit activities, compile and distribute audit reports and complete follow-up activities. It is very important for the organisation to have a smooth flow of accounting as it plays a very important role in the development of the organisation. Financial Managers or any person who deals with Accounts need to see that the company accounts are very updated and are free from any risks that can become a problem during the time of Auditing. Objectives By the end of the course, participants will be able to: Efficiently dealing with senior leaders with confidence Effective Contribution and Strategically Analysing and Auditing towards business success Analysing and Evaluating as an effective internal audit leader How to manage key relationships with the audit committee Practical methods for managing the audit committee and senior management Describing the significance to help maximize the contribution to their organization

Search By Location
- Auditor Courses in London
- Auditor Courses in Birmingham
- Auditor Courses in Glasgow
- Auditor Courses in Liverpool
- Auditor Courses in Bristol
- Auditor Courses in Manchester
- Auditor Courses in Sheffield
- Auditor Courses in Leeds
- Auditor Courses in Edinburgh
- Auditor Courses in Leicester
- Auditor Courses in Coventry
- Auditor Courses in Bradford
- Auditor Courses in Cardiff
- Auditor Courses in Belfast
- Auditor Courses in Nottingham