- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
690 Associate courses in Downpatrick delivered Live Online
Aruba Mobility Fundamentals, Rev. 20.11
By Nexus Human
Duration 3 Days 18 CPD hours This course is intended for Typical candidates for this course are IT Professionals who deploy small-to-medium scale enterprise network solutions based on Aruba products and technologies. Overview After you successfully complete this course, expect to be able to: Explain how Aruba's wireless networking solutions meet customers' requirements Explain fundamental WLAN technologies, RF concepts, and 802.11 Standards Learn to configure the Mobility Master and Mobility Controller to control access to the Employee and Guest WLAN Control secure access to the WLAN using Aruba Firewall Policies and Roles Recognize and explain Radio Frequency Bands and channels, and the standards used to regulate them Describe the concept of radio frequency coverage and interference and successful implementation and diagnosis of WLAN systems Identify and differentiate antenna technology options to ensure optimal coverage in various deployment scenarios Describe RF power technology including, signal strength, how it is measured and why it is critical in designing wireless networks Learn to configure and optimize Aruba ARM and Client Match and Client Insight features Learn how to perform network monitoring functions and troubleshooting This course teaches the knowledge, skills and practical experience required to set up and configure a basic Aruba WLAN utilizing the OS 8.X architecture and features. Using lecture and labs, this course provides the technical understanding and hands-on experience of configuring a single Mobility Master with one controller and AP Aruba WLAN. Participants will learn how to use Aruba hardware and ArubaOS to install and build a complete, secure controller network with multiple SSIDs. This course provides the underlying material required to prepare candidates for the Aruba Certified Mobility Associate (ACMA) certification exam. WLAN Fundamentals Describes the fundamentals of 802.11, RF frequencies and channels Explain RF Patterns and coverage including SNR Roaming Standards and QOS requirements Mobile First Architecture An introduction to Aruba Products including controller types and modes OS 8.X Architecture and features License types and distribution Mobility Master Mobility Controller Configuration Understanding Groups and Subgroups Different methods to join MC with MM Understanding Hierarchical Configuration Secure WLAN configuration Identifying WLAN requirements such as SSID name, encryption, authentication Explain AP groups structure and profiles Configuration of WLAN using the Mobility Master GUI AP Provisioning Describes the communication between AP and Mobility controller Explain the AP booting sequence and requirements Explores the APs controller discovery mechanisms Explains how to secure AP to controller communication using CPSec Describes AP provisioning and operations WLAN Security Describes the 802.11 discovery, authentication and association Explores the various authentication methods, 802.1x with WPA/WPA2, Mac auth Describes the authentication server communication Explains symmetric vs asymmetric Keys, encryption methods WIPS is described along with rogue discovery and protection Firewall Roles and Policies An introduction into Firewall Roles and policies Explains Aruba?s Identity based Firewall Configuration of Policies and Rules including aliases Explains how to assign Roles to users Dynamic RF Management Explain how ARM calibrates the network selecting channels and power settings Explores OS 8.X Airmatch to calibrate the network How Client Match and ClientInsight match steers clients to better APs Guest Access Introduces Aruba?s solutions for Guest Access and the Captive portal process Configuration of secure guest access using the internal Captive portal The configuration of Captive portal using Clearpass and its benefits Creating a guest provisioning account Troubleshooting guest access Network Monitoring and Troubleshooting Using the MM dashboard to monitor and diagnose client, WLAN and AP issues Traffic analysis using APPrf with filtering capabilities A view of Airwaves capabilities for monitoring and diagnosing client, WLAN and AP issues Additional course details: Nexus Humans Aruba Mobility Fundamentals, Rev. 20.11 training program is a workshop that presents an invigorating mix of sessions, lessons, and masterclasses meticulously crafted to propel your learning expedition forward. This immersive bootcamp-style experience boasts interactive lectures, hands-on labs, and collaborative hackathons, all strategically designed to fortify fundamental concepts. Guided by seasoned coaches, each session offers priceless insights and practical skills crucial for honing your expertise. Whether you're stepping into the realm of professional skills or a seasoned professional, this comprehensive course ensures you're equipped with the knowledge and prowess necessary for success. While we feel this is the best course for the Aruba Mobility Fundamentals, Rev. 20.11 course and one of our Top 10 we encourage you to read the course outline to make sure it is the right content for you. Additionally, private sessions, closed classes or dedicated events are available both live online and at our training centres in Dublin and London, as well as at your offices anywhere in the UK, Ireland or across EMEA.

This award introduces the critical concepts associated with AI and explores its relationship with the systems and processes that make up the digital ecosystem. It explores how AI can empower organisations to utilise Big Data through the use of Business Analysis and Machine Learning, and encourages candidates to consider a future vision of the world that is powered by AI.

QA Level 2 Award In Principles Of COSHH (RQF) Face to Face: Half-day course Virtual Classroom: Spread over 2 sessions of 2½ hr duration COSHH: Control of Substances Hazardous to Health Regulations 2002 Hazardous substances are everywhere, and COSHH assessments have to be undertaken in all businesses Run in an engaging and interactive way - see the video below Course Contents: Legal requirements relating to hazardous substances in the workplace Employer duties Employee duties Consequences of non-compliance Communicating safety information The different forms that hazardous substances can take Effects that may arise after exposure to hazardous substances The different routes that hazardous substances can enter the body How risk assessments reduce accidents and ill health at work The five step process of risk assessment The control hierarchy in relation to the safe use, handling, transporting, storage and disposal of substances hazardous to health Information to refer to when identifying and using hazardous substances Procedures for dealing with an incident involving hazardous substances Benefits of this course: COSHH stands for the 'Control of Substances Hazardous to Health' and falls under the Control of Substances Hazardous to Health Regulations 2002 This half day course is for all those who work, or wish to work, in a workplace that works with hazardous substances, such as in healthcare, in the manufacturing sector, cleaners, transport, utilities and even office environments Hazardous substances are everywhere, and COSHH assessments have to be undertaken in all businesses Candidates will learn about the legal requirements relating to hazardous substances, how risk assessments contribute to the safe use of hazardous substances, as well as the precautions and procedures required to ensure that the risks associated with hazardous substances are properly controlled Accredited, Ofqual regulated qualification: This QA Level 2 Award in Principles of COSHH (RQF) Course is a nationally recognised, Ofqual regulated qualification accredited by Qualsafe Awards.This means that you can be rest assured that your Principles of COSHH Certificate fulfills the legal requirements and is a very good way to make sure you and your employees are trained appropriately.The Ofqual Register number for this course is 603/0775/4

Quality Systems for Research Laboratories
By Research Quality Association
Course Information This highly interactive course will provide guidance on why and how to implement a quality system successfully into the research laboratory. By doing so, you will position your innovation for the success it deserves. But leave things as they are and there is a good chance that your science will not realise its full potential should success, and its consequences, come your way. A quality system in your research laboratory is the most effective and efficient way to: Help scientists work more efficiently Ensure discoveries can be defended Protect the value of intellectual property. This course is particularly aimed at those working in early phase research environments which are not constrained by the regulatory requirements of the Good Practice regulations but are producing intellectual property, testing and/or products for the therapeutic market. For organisational reasons, rather than regulatory ones, this is a place where you need to get it right. The programme is delivered by leaders in the field who, quite simply, ‘have done it’. Whether delegates are at senior management level seeking strategic direction, a laboratory head wishing to deliver science that will stand the test of time or a quality professional thrown in at the deep end, this course will provide key insight and practical guidance to underpin future success. Based on risk based systems, tried and tested over many years in the workplace, the programme will help delegates to define, train, implement and monitor the quality of their research, irrespective of field or discipline. Delegates will learn how to help position their organisation for success. Course content: Delegates will be guided thoughtfully through each key component of the process in a stimulating learning environment. The course probes all avenues of the research quality arena, from an initial understanding of the cultural aspects of the scientific discovery environment, to managing quality in outsourced research programmes. Computer systems and e-data security in the research environment will be discussed and pragmatic solutions described to help manage the ballooning cloud of e-data. In addition, the ever blurring boundary between the regulated and non-regulated research environments will be discussed and delegates given perspective on future developments in the field. With this knowledge, delegates will be able to get it ‘right first time’. Is this course for you? The course is designed for all those involved in the research laboratory quality arena and it has been tailored to meet the needs of scientific management, bench scientists and quality professionals alike. Delegates get immediate access to highly experienced tutors who will share their wisdom and insights in an area where few others have been successful. The course is linked with the RQA guidance which builds on years of experience and forms the foundation of the programme. Tutors Tutors will be comprised of (click the photos for biographies): Louise Handy Director, Handy Consulting Ltd Sandrine Bongiovanni Associate Director in Research and Quality Compliance, Novartis Programme Please note timings may be subject to alteration. Day 1 09:00 Registration 09:10 Welcome and Introductions 09:20 History and Overview of the Field Examples of business and regulatory risks and the consequences of low quality in research. A look at the standards and guidelines that exist. 10:00 The Culture, the Politics and the Scientist's Perspective Understanding research environments, the drivers and the challenges. 10:30 Break 10:45 Workshop - Risk Management Thinking about risk management and prioritisation. Looking at the critical factors for the implementations of a successful quality system. 12:15 Workshop - Feedback 12:45 Lunch 13:45 Personnel, Plans, Procedures, Facilities, Equipment, Materials and Reagents Looking at planning the work, defining procedures in a way which promotes robust science without compromising brilliance and ensuring that all these elements are demonstrably fit for their intended purpose. 14:30 Workshop - Assay Validation How much validation is required at what stage? What do we need to validate an assay? 15:00 Workshop - Feedback 15:15 Research, Work Records, Archives and Research Review Data and records which are accurate, attributable, legally attestable and safe to permit reconstruction experiments and studies. Looking at aspects of the work where there is a chance to review, correct or improve the science, the data and the processes. 16:15 Continual Improvement and Quality Systems Reviewing implementation of a quality system, finding opportunities for improvement, understanding culture change. 16:45 Questions and Answers 17:00 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Develop

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

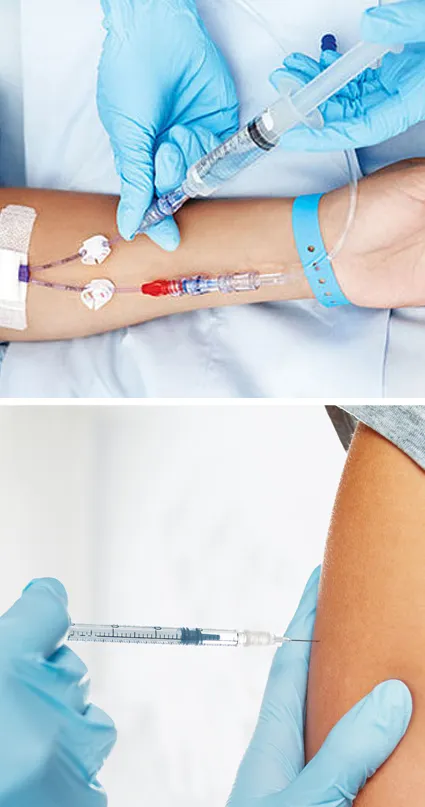
THIS COURSE PACKAGE INCLUDES: 1: PERIPHERAL I.V. CANNULATION - IV THERAPIES COURSE (GPT008) 2: VACCINATION / INJECTION COURSE (GPT601) Learn how to administer injectables and intravenous therapies ... FAST-TRACK YOUR AESTHETICS TRAINING WITH OUR COMPLETE TRAINING PACKAGE 20% Multi-Course Discount Cover all stages from Level 1 through to Level 4 (FDSc) Cover your theory training online Complete your advanced practical training in 1 day Practical training in Classroom or Virtual Classroom Comprehensive Practise@Home training kits for VC Awards 2 accredited qualifications Dual Accreditations for all courses Covers all steps required to safely perform injectables Covers all steps required to safely perform IV therapies Practise IV on artificial arm with fake blood Practise injection techniques on realistic injection pads Learn beginner to advanced skills and techniques Basic understanding of English language required OPEN TO ALL APPLICANTS
The course is relevant to anyone requiring an understanding of the use of Agile or looking to adopt it. This includes, but is not limited to, organisational leaders and managers, marketing executives and managers, and/or all professionals working in an Agile environment, including software sesters, developers, business analysts, UX designers, project management office (PMO), project support and project coordinators.