- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
The Central Heating Controls Wiring and Fault Finding course is a two day-day short course aimed at anyone involved in the construction, commissioning, inspection & testing or maintenance of central heating electrical control systems. The Central Heating Controls Wiring and Fault Finding course covers all the commonly used control systems in use today and is focused on the ‘practical’ construction and commissioning along with the relevant fault finding techniques. The Central Heating Controls Wiring and Fault Finding course requires an understanding of electrical principles and cable termination skills. A requirement of this course is the successful completion of their Essential Electrics examination prior to sitting the central heating controls wiring & fault finding examinations. Please ring if clarification is needed on this point. There are two 20 minute open book examinations and the associated practical assessments. A third examination and associated assessment will be required for candidates not holding the Essential Electrics unit. The Central Heating Controls Wiring and Fault Finding course comprises of: Short theory sessions introducing the conventional wiring systems, ‘Y’ Plan, ‘S’ Plan, ‘C’ Plan, Etc. Detailed practical workshop sessions, undertaking the construction, testing and commissioning of systems Fault finding and maintenance tasks performed on working systems Examination and practical assessment preparation ready for the assessments Evaluation of the system control function (why and how it works) Each student will work on their own system, and will have plenty of time to absorb and understand how each systemworks. Advise will be given on suitable ‘tooling’ and test equipment. These are nationally recognizable qualifications which are fast becoming an essential requirement for this type of work. The course costs include comprehensive course notes and examination entry fees.

Principles of Manual Handing
By Pathways Training
This course outlines exactly what constitutes manual handling and covers the regulations and legislation that apply to manual handling tasks. It then goes on to cover safe handling techniques and how to develop good habits in relation to manual handling. It finishes off by introducing some practical solutions and the use of mechanical aids. Please note that this is an awareness course only, if your duties include manual handling you will also need further practical training, you can get in touch with us to arrange this. Learning Objectives Define Manual Handling and state the correct technique and application of effective Manual Handling. Understand the relevant legislation and comply with the regulations. Recognise safe handling techniques and be able to develop good habits. Evaluate every manual situation you encounter, recognising risks and when to use mechanical aids. Understand the consequences on the body of incorrect handling and the dangers associated. State the professionals most at risk from incorrect handling techniques.

Awareness of First Aid for Mental Health
By Madeleys First Aid Plus
🧠 Mental Health Matters — In Every Workplace 💬 Did you know? 📊 1 in 4 people in the UK experience a mental health condition each year 💼 1 in 6 employees face common mental health challenges at work This 4-hour Mental Health Awareness qualification helps break the silence and stigma by equipping you to: ✅ Recognise signs of mental health conditions 🗣️ Start supportive conversations 📍 Signpost to professional help 🌿 Understand and manage workplace stress You won’t be diagnosing — but you will be making a difference. 📅 Join us in creating safer, more supportive workplaces. #MentalHealthAwareness #WorkplaceWellbeing #EndTheStigma #SupportEachOther #MentalHealthTraining #BeThere

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

Intermediate Professional Practice
By CORE theatre arts training
CORE is a professional level training company in physical theatre and acting with a solid emphasis on the classical “Theatre Apprenticeship” training model. With 30+ years experience and using proven training methods, combined with our unique mentoring approach, we develop the whole person, in order to equip our trainees to identify their God-given calling and use their gifts to impact culture and society for good. EQUIPPING YOU FOR LIFE One of our unique qualities lies in our ability to identify your strengths, regardless of your experience and training, and develop you further. You will be challenged, receive practical skills and confidence to make your next step, whether you want to take qualifications with Trinity College London, train as a teacher or get an agent as you step into the challenging world of the performing arts. Training groups are kept small to ensure individual attention and you receive a wealth of transferrable skills to equip you for life in the workplace. OUR UNIQUE APPROACH CORE's uniqueness lies in our attention to individual coaching, personal and professional development whilst maintaining a culture of excellence and rigorous discipline. We engage trainees in professional productions for on and off stage experience, and in collaborative works with other artists and musicians. Admission is by audition only (min age 16) no educational qualifications required, as admission is based on Potential, Passion and Perseverance that are essentials required to thrive in this industry and in life!

Level 4 Award in Immediate Life Support (ILS) Course
By NR Medical Training
The Level 4 Award in Immediate Life Support (RQF) is ideal for a wide range of healthcare professionals. This includes doctors, dental professionals, medical students, nurses, midwives, and physiotherapists who need an ILS qualification for their registration with regulatory bodies like the GMC, GDC, NMC, and HCPC. It's also perfect for those looking to advance in their careers or needing an ILS certification for new job opportunities.

C10M11 – Hazardous Areas Overview for Fire Detection and Alarm Systems (Classroom)
5.0(2)By Ember Compliance
Learners will be given a broad overview of hazardous areas with a particular emphasis on the requirements for intrinsically safe fire detection and alarm systems.

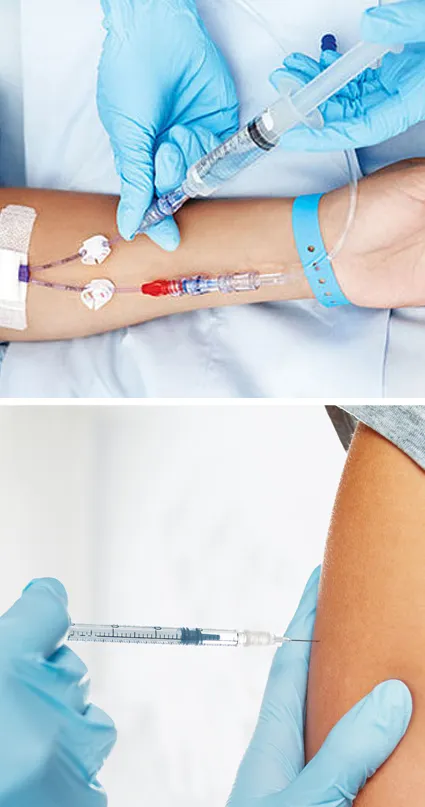
THIS COURSE PACKAGE INCLUDES: 1: PERIPHERAL I.V. CANNULATION - IV THERAPIES COURSE (GPT008) 2: VACCINATION / INJECTION COURSE (GPT601) Learn how to administer injectables and intravenous therapies ... FAST-TRACK YOUR AESTHETICS TRAINING WITH OUR COMPLETE TRAINING PACKAGE 20% Multi-Course Discount Cover all stages from Level 1 through to Level 4 (FDSc) Cover your theory training online Complete your advanced practical training in 1 day Practical training in Classroom or Virtual Classroom Comprehensive Practise@Home training kits for VC Awards 2 accredited qualifications Dual Accreditations for all courses Covers all steps required to safely perform injectables Covers all steps required to safely perform IV therapies Practise IV on artificial arm with fake blood Practise injection techniques on realistic injection pads Learn beginner to advanced skills and techniques Basic understanding of English language required OPEN TO ALL APPLICANTS
Search By Location
- Associate Courses in London
- Associate Courses in Birmingham
- Associate Courses in Glasgow
- Associate Courses in Liverpool
- Associate Courses in Bristol
- Associate Courses in Manchester
- Associate Courses in Sheffield
- Associate Courses in Leeds
- Associate Courses in Edinburgh
- Associate Courses in Leicester
- Associate Courses in Coventry
- Associate Courses in Bradford
- Associate Courses in Cardiff
- Associate Courses in Belfast
- Associate Courses in Nottingham