- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
5287 AR courses in Cardiff delivered Live Online
M.D.D ANXIETY AND STRESS MANAGEMENT COUNSELLING PACKAGE (SELF IMPROVEMENT)
4.9(27)By Miss Date Doctor Dating Coach London, Couples Therapy
Address root cause Implement healing strategies Life coaching Emotional intelligence guidance Emotional management programme CBT or modern stress relief techniques Support coach Twice a week 1 hour X 5 sessions Stress Management Counselling is a type of therapy that can help individuals who are struggling with the overwhelming effects of stress. Our professional counsellors provide guidance and support to help clients develop effective coping strategies to manage their stress levels. Our goal is to empower clients to take control of their lives and improve their overall well-being. Whether you’re dealing with work-related stress, relationship issues, or any other stressors, our stress management counselling sessions can provide a safe and confidential space for you to explore your thoughts and feelings. Contact us today to schedule an appointment and start your journey towards a healthier, happier life. https://relationshipsmdd.com/product/anxiety-and-stress-package/

ONLINE. Icon Painting Course - Beginners. With live demonstrations.
4.4(18)By Edinburgh School of Icon Painting
We are excited to announce the Online version of the Step by Step Course. It will include live demonstrations of the process that you will then follow. Unique opportunity to be guided step by step and experience meditative practice of icon painting.
Level 3 Assessor Certificate CAVA Course
By Canary Wharf Academy
Become a proficient assessor with our Level 3 Assessor CAVA course, designed to equip you with the essential skills to assess vocational competence effectively. Whether you're evaluating skills in a workshop, classroom, or training environment, this comprehensive course prepares you to guide learners through their learning journey with precision and feedback. Course Outline: Unit 301: Understanding the Principles and Practices of Assessment Unit 302: Assess Occupational Competence in the Work Environment Unit 303: Assess Vocational Skills, Knowledge, and Understanding Entry Requirements: No formal prerequisites are needed, but candidates should work in or aspire to quality assurance roles. This course is a solid foundation for those venturing into quality assurance responsibilities. Access to two learners is required for the full Award. Course Assessment: Under the guidance of your assessor, you'll compile a portfolio of evidence throughout the course. Your assessor will mark this portfolio, which will be internally quality-assured by the centre and authority. Course Fee: Online/Distance Learning: £359.99 Zoom Classroom-based Course: £479.99 Corporate or Group Booking: Benefit from special discounts on corporate or group bookings, allowing our experienced trainers to deliver tailored training at your location. How to Book: Book online via PayPal, debit/credit card, invoice, or bank transfer. You can also book over the phone or visit our office for assistance. Instant booking confirmation will be sent via email. Need Assistance? For any queries or assistance, our dedicated team is just a phone call or email away. We're here to support you every step of the way. Contact us today to embark on your journey towards becoming a certified assessor. Book Now

CDS: NEW CUSTOMS FOR EXPORTS/IMPORTS
By Export Unlocked Limited
Are you a customs agent/ Importer ? Or does your company use customs agents and intermediaries to help you trade with the EU and the Rest of the World? If so, you need to know how to meet customs requirements fast and efficiently now the new customs declaration system CDS is in place. We can help.

M.D.D COUPLES SERIOUS ARGUMENT PACKAGE (ARGUMENT WITH PARTNER)
4.9(27)By Miss Date Doctor Dating Coach London, Couples Therapy
This package is for a couple Diffuse and calm down situation Speak to both parties separately Mediate situation Rationalise through the problems Address the area of contention Couples therapy Ascertain issues Advice on future communications Resolution 2 30min sessions with each party https://relationshipsmdd.com/product/couples-serious-argument-package/

3ds Max Essential Training Course
By ATL Autocad Training London
Course Title: 3ds Max Essential Training Course Perfect for novice 3ds Max users, our Essentials Training Course, hosted by an Autodesk Certified Trainer, equips you with core skills for creating 3D models and animations. Click here for more info: Website Duration: 16 hours Method: 1-on-1, Personalized Attention, Tailored Content, Flexible Pace, Individual Support Schedule: Tailor your own schedule by pre-booking a convenient hours, available from Mon to Sat between 9 am and 7 pm. Course Highlights: Comprehensive Learning: Covering 3ds Max from the basics, including interface navigation and customization, to essential skills for professional 3D modeling and animations. Expert Guidance: Certified tutors and industry experts provide personalized attention, ensuring a deep understanding of 3D concepts. Flexible Scheduling: Tailor the learning experience to your pace and preferences, with one-on-one sessions available from Monday to Sunday. Interactive Learning: Engage in hands-on exercises and practical projects, enhancing your skills in a real-world context. Job Opportunities: Upon completion, students can pursue careers as: 3D Modelers: Creating detailed 3D models for games, movies, or architectural visualization. Animation Artists: Designing engaging animations for various media platforms. Visual Effects (VFX) Artists: Working on special effects for films, TV shows, and commercials. Architectural Visualizers: Producing realistic architectural renders for construction and design projects. Game Designers: Developing immersive game environments and characters. Recommended Reading: "3ds Max 2022 for Beginners" by CADFolks: A beginner-friendly guide covering fundamental concepts and techniques in 3ds Max. "Mastering Autodesk 3ds Max 2022" by Nicholas Boughen: A comprehensive resource for mastering advanced features and workflows in 3ds Max. "The Animator's Survival Kit" by Richard Williams: A classic animation guide providing valuable insights for aspiring animators. These books offer valuable knowledge and techniques, complementing the skills learned in the course and aiding in future career endeavors. Upon completing the 3ds Max Basic to Fundamentals Training Course, participants will: Master 3ds Max Tools: Gain a deep understanding of the software interface, tools, and navigation, enabling efficient 3D modeling and animation. Create Complex Models: Develop proficiency in creating intricate 3D models, including objects, characters, and architectural elements, using various modeling techniques. Apply Realistic Textures: Learn to apply textures, maps, and materials to enhance the realism of 3D models, understanding diffuse, bump, and reflective materials. Perfect Lighting and Rendering: Acquire skills in setting up various lights, environment lighting, and global illumination for realistic renderings, optimizing rendering settings for quality output. Animate with Precision: Master keyframe animation, path animation, and constraints, bringing 3D scenes to life with smooth and accurate motion. Understand Dynamics and Simulations: Explore basic dynamics and simulations, including particle systems, creating dynamic and interactive 3D environments. Problem-Solve Creatively: Develop problem-solving skills for common challenges in 3D design, using innovative techniques and tools. Professional Project Execution: Apply learned skills to real-world projects, ensuring a professional approach to 3D modeling, animation, and visualization. By the end of the course, participants will be equipped with the knowledge and skills to confidently create visually stunning 3D models, animations, and visualizations, making them proficient 3ds Max users ready for diverse industry applications. Course Highlights: Comprehensive Learning: Covering 3ds Max from the basics, including interface navigation and customization, to essential skills for professional 3D modeling and animations. Expert Guidance: Certified tutors and industry experts provide personalized attention, ensuring a deep understanding of 3D concepts. Flexible Scheduling: Tailor the learning experience to your pace and preferences, with one-on-one sessions available from Monday to Sunday. Interactive Learning: Engage in hands-on exercises and practical projects, enhancing your skills in a real-world context. Job Opportunities: Upon completion, students can pursue careers as: 3D Modelers: Creating detailed 3D models for games, movies, or architectural visualization. Animation Artists: Designing engaging animations for various media platforms. Visual Effects (VFX) Artists: Working on special effects for films, TV shows, and commercials. Architectural Visualizers: Producing realistic architectural renders for construction and design projects. Game Designers: Developing immersive game environments and characters. Recommended Reading: "3ds Max 2022 for Beginners" by CADFolks: A beginner-friendly guide covering fundamental concepts and techniques in 3ds Max. "Mastering Autodesk 3ds Max 2022" by Nicholas Boughen: A comprehensive resource for mastering advanced features and workflows in 3ds Max. "The Animator's Survival Kit" by Richard Williams: A classic animation guide providing valuable insights for aspiring animators. These books offer valuable knowledge and techniques, complementing the skills learned in the course and aiding in future career endeavors. Receive ongoing email support for a lifetime. Access comprehensive handouts and valuable documents. Explore flexible financial support choices, including installment plans and funding through job center plus and DWP (Contact us for specifics). Rest assured with our money-back guarantee: If you're dissatisfied after your initial session, we address your concerns and provide a refund if necessary (Terms and conditions may apply). Get help with computer optimization to enhance software performance in Autocad, 3ds Max, and Photoshop on both PC platforms. Benefit from our industry connections, facilitating portfolio promotion and job opportunities.
Basics to Essential Photoshop Skills Course
By ATL Autocad Training London
Basics to Essential Photoshop Skills Course, Master retouching, layers, color, correction, shapes, and symbols. Delve into graphic design, photo editing, and digital art. Elevate your skills from beginner to pro, gaining expertise in UX strategies, ensuring your websites are impactful and user-friendly. Click here for more info: Website Duration: 16 hours Method: Personalized 1-on-1 sessions ensure individual attention. Schedule: Customize your learning with pre-booked sessions available Monday to Saturday, from 9 am to 7 pm. Module 1: Introduction to Photoshop (2 hours) Understanding the Photoshop interface and workspace Navigating tools, panels, and menus Essential keyboard shortcuts for efficient workflow Introduction to different file formats and their uses Module 2: Basic Image Editing Techniques (2 hours) Cropping, resizing, and straightening images Color adjustments and corrections using adjustment layers Removing blemishes and distractions with healing tools Introduction to layers and blending modes Module 3: Advanced Image Manipulation (2 hours) Creating composite images with layer masks Utilizing advanced selection tools for precise editing Applying filters and special effects for creative enhancements Mastering text and typography in Photoshop Module 4: Graphic Design and Layout (2 hours) Designing banners, posters, and social media graphics Working with shapes, gradients, and patterns Creating visually appealing typography compositions Introduction to vector graphics and custom shapes Module 5: Web and UI Design (2 hours) Designing user interfaces for websites and applications Creating web-ready graphics and optimizing images Prototyping interactive elements and buttons Designing responsive layouts for various devices Module 6: Introduction to 3D and Animation (2 hours) Creating 3D objects and text Basic 3D manipulation and lighting effects Introduction to animation with the Timeline panel Exporting animations and interactive multimedia Module 7: Project-Based Learning (2 hours) Applying learned techniques to real-world projects Designing a digital artwork, website mockup, or social media campaign Receiving instructor feedback for skill refinement Final presentation of completed projects and portfolio building Upon completing our personalized Photoshop Mastery course, you'll: Master Tools: Excel in Photoshop's essential and advanced tools, including image manipulation and graphic design techniques. Design Expertise: Develop skills in creating compelling graphics, web layouts, and interactive UI designs. 3D & Animation Skills: Understand 3D manipulation, lighting, and basic animation techniques. Career Opportunities: This course prepares you for roles such as Graphic Designer, Web/UI Designer, Digital Artist, Photo Retoucher, 3D Artist, or Entrepreneur in the design industry. Dive deep into the world of Photoshop with our exclusive 1-on-1 training program. Tailored to your pace and skill level, this course offers a comprehensive understanding of Photoshop's essentials and advanced features. From image editing to graphic design and 3D manipulation, master Photoshop with personalized attention and hands-on guidance. Key Details: Personalized Attention: Enjoy dedicated 1-on-1 sessions with an experienced Photoshop instructor, ensuring focused learning and personalized guidance. Tailored Curriculum: The course content is customized based on your goals, allowing you to explore specific areas of interest and address individual challenges. Flexible Scheduling: Schedule sessions at your convenience, accommodating your busy lifestyle. Choose from weekdays or weekends, morning or evening, for a flexible learning experience. Hands-on Learning: Engage in practical, real-time exercises and projects tailored to your interests, reinforcing your skills and boosting confidence. In-depth Exploration: Cover a wide range of topics, including image editing, graphic design, 3D manipulation, web design, and more, delving deep into each area for a thorough understanding. Interactive Q&A: Participate in interactive Q&A sessions during each class, clarifying doubts and gaining valuable insights from your instructor. Lesson Recordings: Access recordings of your sessions for review and reinforcement, ensuring you grasp every concept and technique thoroughly. Ongoing Support: Benefit from continuous email support even after the course completion, receiving guidance on projects and addressing any post-training queries. Solid Foundation: Develop foundational Photoshop skills, mastering essential techniques for image editing and graphic design. Versatile Editing: Learn retouching, color correction, and photo enhancement for professional-quality results. Efficient Workflow: Optimize your work with layer management, non-destructive editing, and time-saving tricks. Creative Mastery: Harness Photoshop's power to create digital art, manipulate images, and design stunning graphics. Real-world Application: Apply skills to diverse projects like photo editing, web design, and social media graphics. Flexible Review: Access recorded lessons for convenient review of specific techniques or workflows. Lifelong Support: Enjoy lifetime email assistance for guidance, questions, and clarifications even after course completion.

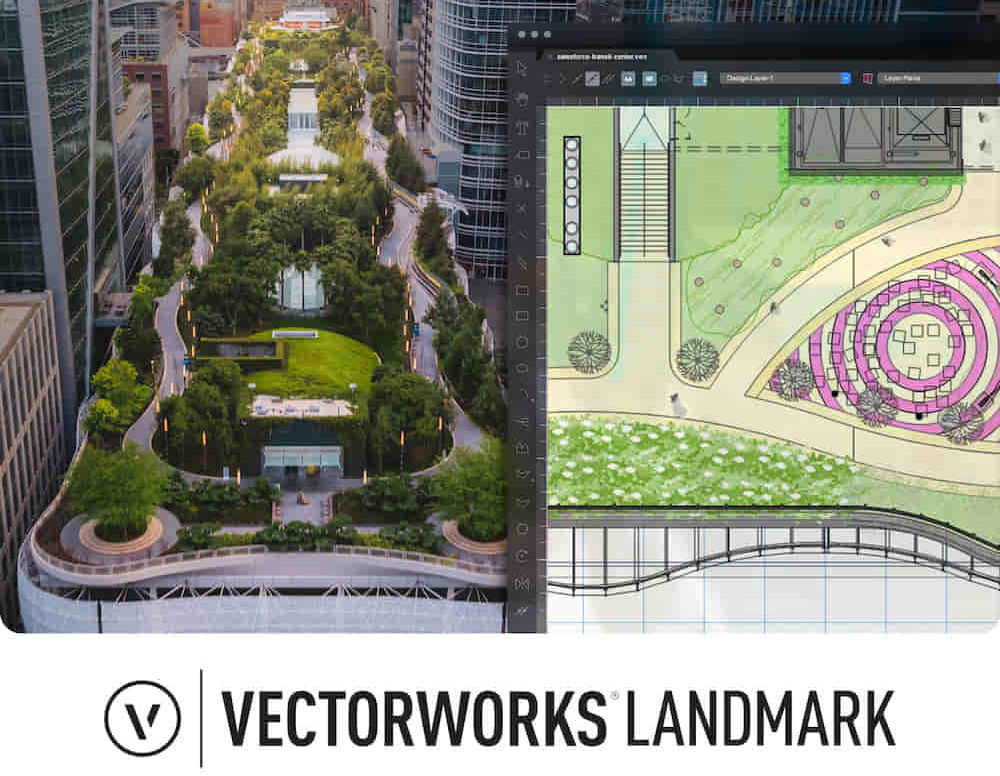
Landmark Training Course With Vectorworks
By ATL Autocad Training London
Who is this course for? Landmark Training Course With Vectorworks. Dive into terrain modeling, planting, irrigation, and site analysis guided by certified tutors. Master these tools for precise landscape designs and effective documentation. Check our Website Enrollment : 1-on-1 Landmark Training. Tailor your schedule. Mon to Sat between 9 am and 7 pm Call 02077202581 to book your slot. Duration: 16 hours. "Split these hours over multiple days as needed for your ideal schedule." Approach: In-person or live online. Landmark Training Course with Vectorworks: Basic to Intermediate Level Course Duration: 16 Hours Embark on a transformative journey with our Landmark Training Course tailored for landscape architects and designers. Over 16 intensive hours, dive deep into Vectorworks Landmark, mastering fundamental and intermediate techniques crucial for comprehensive 2D and 3D landscape design. Craft intricate site analyses, plans, irrigation systems, and elevate your designs with mesmerizing 3D visualizations. Explore custom plant symbols, detailed planting plans, and learn the art of efficient collaboration and customization. Course Highlights: I. Introduction to Vectorworks Landmark (1 hour) Explore Vectorworks Landmark for landscape design Master interface, tool usage, and project management II. Site Analysis and Site Plans (3 hours) Craft detailed site analyses and hardscape designs Work with contours, elevations, and terrain models III. Planting Plans (5 hours) Utilize the plant database for region-specific plant selection Create personalized plant symbols and comprehensive planting plans IV. Irrigation Design (2 hours) Design and edit efficient irrigation systems and zones Integrate irrigation components into site plans V. 3D Visualization (3 hours) Create captivating 3D models with realistic textures and materials Enhance designs with advanced lighting and special effects VI. Customization (1 hour) Tailor the interface for efficient landscape design Create custom object styles and resource libraries VII. Collaboration and Sharing (1 hour) Seamlessly import/export data from other platforms Share designs effectively with colleagues and collaborators VIII. Conclusion and Next Steps (1 hour) Review course content comprehensively Receive guidance on further learning resources Engage in a Q&A session and provide valuable feedback Enhance your landscape design expertise and unleash your creativity. Enroll now in our Vectorworks Landmark Basic to Intermediate Training and transform your designs. Download Vectorworks Landmark By the end of the Vectorworks Landmark Training Course, participants will: Understand the key features and functionalities of Vectorworks Landmark for landscape design. Demonstrate proficiency in using essential tools for site analysis, site plans, and hardscape design. Create detailed planting plans, selecting appropriate plants, and understanding their compatibility and growth patterns. Design efficient and effective irrigation systems, including generating irrigation reports. Create 3D visualizations of landscape designs, applying textures, materials, lighting, and special effects. Customize the interface and create personalized object styles and resources. Collaborate and share landscape design drawings with other software users. Have the foundation to pursue further learning and exploration in landscape design using Vectorworks Landmark. Mastering Foundations Begin your project by organizing your files and importing survey data. Learn to sketch existing buildings using Building Shell tools and model neighboring structures with Massing Model. Explore different methods for laying out survey points and marking existing features with precision. Objectives: Review File Organization Techniques Import Survey Data (DWG Import) Create Building Structures with Building Shell Tools Utilize Triangulation and Arc Tool for Precision Master Various Tape Measurement Techniques Elevating Your Design Enhance your survey with detailed ground, existing trees, and fences. Conduct shadow analysis to optimize planting locations. Dive into the Vectorworks Plant tool, your key design companion. Objectives: Develop Detailed Ground Surfaces Incorporate Existing Trees and Fences Design with Railing Fence Tool Conduct Shadow Analysis using Heliodon Tool Utilize Plant Tool Modes for Plant Placement Access Existing Plant Libraries and Customize Plants in 2D/3D Crafting Landscapes Create vibrant plant mixes using Landscape Area tool and apply them across your site models. Design intricate hardscapes, aligning them effortlessly even in complex paving scenarios. Learn to use components for detailed reporting, cut and fill calculations, and precise detailing. Explore custom object creation and site furniture placement. Objectives: Design Landscape Areas and Define Custom Plant Mixes Create Hardscapes and Define Custom Paving Constructions Generate Reports and Tags for Landscape Areas and Hardscapes Access and Manage Objects in Resource Manager Craft Custom Objects and Site Furniture Polished Presentation Present your designs professionally using Sheet layers and viewports. Create Section viewports to cut through your model and Detail viewports to focus on specific areas. Enhance visual appeal with mood boards and annotations, ensuring a refined, detailed presentation. Objectives: Craft Sheet Layers for Presentation Create Plan, Elevation, and Perspective Viewports Generate Section and Detail Viewports Annotate Viewports for Clear Communication Incorporate Images and Plant Reports for Comprehensive Presentations Master Vectorworks Landmark: Gain expertise in essential and advanced 2D/3D landscape design tools for precision and efficiency. Boost Efficiency: Learn time-saving techniques and workflows tailored to Vectorworks Landmark, enhancing productivity. Versatile Landscape Skills: Develop proficiency in site analysis, planting plans, hardscapes, and irrigation systems for diverse projects. Industry-Ready Expertise: Acquire sought-after skills in landscape architecture, paving the way for career growth. Flexible Learning: Access recorded lessons for convenient review and receive lifetime email support for ongoing guidance.
Garden Design Courses with Vectorworks Training Program
By ATL Autocad Training London
Why Choose Garden Design Courses with Vectorworks Training Program? Designed to enhance your skills in crafting stunning garden designs. Led by certified tutors. From 2D layouts to 3D landscapes and detailed plans and visualizations. Check our Website Details: 1-on-1 training. Customize your schedule, available Mon to Sat 9 am and 7 am Call 02077202581 to book your session over the phone. Duration: 16 hours. "You can divide this over multiple days to suit your schedule." Approach: 1-on-1 in-person or live online. Course Duration: 16 hours Course Description: This advanced Vectorworks course is tailored for individuals who already possess basic knowledge of the software and wish to enhance their skills to become proficient users. Participants will learn more advanced 2D and 3D techniques, including advanced 3D modeling, rendering, visualization, and customizing Vectorworks to suit their specific needs and workflows. The course will cover topics such as creating custom tools, working with planting plans, and integrating with AutoCAD. Course Outline: Module 1: Vectorworks Interface and Basic Concepts - Understanding the Vectorworks interface and palettes - Opening, saving, and managing files - Creating and editing objects and shapes - Utilizing Undo/Redo and Snaps for precision - Applying graphic attributes and working with dimensions Module 2: Modeling and Drawing Techniques - Exploring advanced tools and commands in detail - Integrating with AutoCAD and data exchange - Creating, modifying, and editing text - Utilizing callout text tool for annotations - Organizing information using design layers and classes Module 3: Working with Buildings and Models - Utilizing design layers and sheet layers for architectural projects - Assigning objects to layers and classes - Working with viewports and sheet layers for presentation - Drawing buildings, including walls and roofs - Setting up and rendering perspective views Module 4: Creating Site and Landscape Plans - Importing land surveys or architects' files - Drawing site surveys and working with hard landscape elements - Adding and customizing plantings using the plant tool - Editing plant definitions and creating custom plant symbols - Generating plant schedules and reports Module 5: Vectorworks Architect Features - Setting up files and scaling the drawing - Customizing text styles and dimension standards - Working with scanned images and creating site models - Designing floors, doors, and windows using standard Vectorworks elements - Creating and managing title blocks, labels, notes, and keynotes Module 6: Working Drawings and Final Project - Developing the final project with 2D and 3D elements - Applying site modifiers and stairs to the design - Generating printing layouts for presentations - Creating working drawings with detailed plans and elevations Download Vectorworks Trial https://www.vectorworks.net/trial Learning Outcomes: Master Vectorworks Tools: Proficiency in Vectorworks software, specializing in garden design features. Comprehensive Garden Design Skills: Expertise in 2D/3D modeling, plant selection, hardscapes, and irrigation systems. Professional Documentation: Create precise construction documents and visually compelling presentations. Collaboration and Project Management: Understand collaborative workflows, project management, and client communication. Job Opportunities: Landscape Designer/Architect: Design aesthetically pleasing and functional gardens for residential and commercial spaces. Garden Consultant/Horticulturist: Provide expert advice on plant selection, garden health, and sustainable practices. Entrepreneur/Educator: Start your own garden design business or teach garden design principles and techniques. Elevate your career with the Vectorworks Garden Design Course! Vectorworks Garden Design Mastery Course Course Enrollment Details: Unlock a personalized learning journey with our flexible 1-on-1 training sessions. Customize your schedule by reserving a time slot at your convenience, available Monday to Saturday between 9 a.m. and 7 p.m. Call 02077202581 to book your session over the phone. Training Duration: 16 hours. "You can divide these 16 hours over multiple days to suit your schedule." Training Approach: Experience tailored 1-on-1 sessions, either in-person or live online, providing individualized attention, customized content, flexible pacing, and comprehensive support. Live online 1-on-1 sessions via Zoom are also available. Course Overview: The Vectorworks Garden Design Mastery Course is meticulously crafted to empower participants with the expertise needed to craft exquisite garden designs using Vectorworks software. Guided by certified tutors and industry professionals, this program delivers a profound understanding of Vectorworks tools specifically tailored for garden design, enabling you to transform your landscaping ideas into breathtaking realities. Throughout the course, delve into the specialized features and functionalities of Vectorworks tailored for garden design. From 2D layouts to intricate 3D landscapes, master the art of utilizing Vectorworks to create detailed plans, visualize designs, and produce impeccable documentation for your garden projects. Tailored Training Excellence: Personalized One-to-One Guidance: Experience individualized coaching with undivided attention and customized instruction. Flexible Scheduling: Choose your preferred training slots, tailored to your schedule, even on weekends and late evenings. Post-Course Support and Comprehensive Materials: Access free online support post-training and receive detailed PDF notes and handouts for effective learning. Recognition of Achievement: Earn a Certificate of Attendance upon course completion, validating your expertise. Affordable Learning Solutions: Enjoy budget-friendly training rates without compromising on the quality of education. Tech Assistance and Referral Benefits: Receive software setup support and unlock referral discounts by recommending friends. Group Learning Perks and Tailored Courses: Avail special discounts for group sessions and experience customized training designed just for you. Elevate your skills affordably with our flexible schedules and personalized support.

AutoCAD 2D Basics to Advanced Course
By ATL Autocad Training London
Who is this course for? AutoCAD 2D Basics to Advanced Course. Click here for more info: Website This course enables you to learn the skills in the CAD. Upon completion, you'll proficiently edit and create 2D drawings, utilizing advanced features like Paperspace and Block Attributes for increased efficiency. 1-on-1 sessions. Our booking are available Mon to Sat, 9 am to 7 pm Duration: 16 hours, which you can flexibly distribute across as many days you want. Approach: In-person or live online training. AutoCAD Basics to Advanced Level Training Course Outline. Fundamental Concepts: Introduction to AutoCAD interface, commands, and tools. Managing drawings and creating basic shapes. Basic modification techniques like erase, move, rotate, and scale. Intermediate Techniques: Working with layers and adjusting properties. Creating and modifying text and dimensions. Introduction to blocks and attributes. Advanced Topics: Advanced object modification techniques such as fillet, chamfer, trim, and extend. Utilizing grips for object manipulation. Creating and editing polylines and splines. Course Highlights: Engaging exercises and projects for skill reinforcement. Access to Q&A and troubleshooting support. Proficiency in AutoCAD 2D for basic to intermediate drawings. Topics Covered: Drawing techniques encompassing various shapes. Inquiry tools for measurement and selection. Modification commands for object manipulation. Layer management and attributes. Annotation and dimensioning. Hatching objects and working with reusable content. Layouts and viewports for better organization. Annotating drawings effectively. Polylines, splines, ellipses, and tables. Plotting drawings and creating templates. Multi-lines, revision clouds, and wipeout objects. Working with point objects and calculations. Creating and managing templates. Annotation scaling and text manipulation. Dimensioning and geometric annotations. Dynamic blocks and attributes. Layer management best practices. Introduction to sheet sets and their properties. Utilizing fields and attributes in sheet sets. Working with tables, table styles, and advanced tables. Exporting and importing tables. Creating tables linked to external data. Download AutoCAD Software https://www.autodesk.co.uk What Will I Gain from this Course? Throughout this course, you will develop expertise in the following areas: CAD layering techniques Text and dimension style manipulation Proficiency in creating and editing 2D drawings Mastery of attributed block creation and editing Efficient utilization of Paperspace Dynamic input and grips utilization This comprehensive training program not only provides you with an advanced comprehension of 2D drawing in AutoCAD but also offers a fundamental introduction to 3D drawing concepts. Upon completion of this course, you will possess the skills to: Swiftly generate schematics, parts, and assemblies Enhance efficiency by reusing content and customizing tool palettes Save time through the use of dynamic blocks, which can be edited in place Gain full control over your viewports and layouts Is This Course Right for Me? This advanced AutoCAD 2D training assumes that you are already a proficient AutoCAD user. We recommend prior attendance of our Introduction and/or Intermediate AutoCAD 2D training courses or practical experience in the topics covered in those courses. If you have any doubts about the suitability of this course for your skill level, please feel free to contact us, and we will be happy to assist you. In this course, you will gain proficiency in utilizing essential features of AutoCAD (Computer Aided Design) to enhance the efficiency of producing and editing 2D CAD drawings. You will acquire expertise in layering, text and dimension styles, the creation and modification of attributed blocks, and effective use of Paperspace. Additionally, you will master dynamic inputs, grips, and the art of renaming and purging. This flexible course grants you 16 hours to complete, typically spanning 4 to 5 days. It serves as a valuable continuation of skills acquired from the CAD: AutoCAD 2D Essentials course or as an opportunity to expand your existing AutoCAD knowledge. Upon course completion, you will possess the ability to efficiently create and modify 2D drawings, leveraging advanced features like Paperspace and Block Attributes. Your instruction will be provided by an Autodesk-certified instructor with industry experience." 1. All-encompassing course designed to accommodate both novices and advanced users. 2. Addresses essential functions and advanced methodologies in AutoCAD. 3. Enhances proficiency in creating precise and professional 2D drawings. Interactive instruction guided by seasoned educators. Benefit from on-demand access to lesson recordings for convenient revision. Receive ongoing support through lifetime email and phone assistance. Post-course Assistance: Enjoy continuous support via lifetime email and phone assistance. Should you encounter any challenges or queries after the course, we are readily available to address your concerns via email or phone calls."