- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
630 Courses about Healthcare in Cardiff delivered On Demand
VAL03: Commissioning and Installation Qualification
By Zenosis
Before equipment can be used routinely in production, it must first be commissioned and, if necessary, undergo Installation Qualification (IQ). This module describes commissioning and IQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
VAL04: Operational and Performance Qualification
By Zenosis
Having undergone Installation Qualification, before equipment can be used routinely in production, it needs to undergo Operational Qualification (OQ) and Performance Qualification (PQ). This module describes OQ and PQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
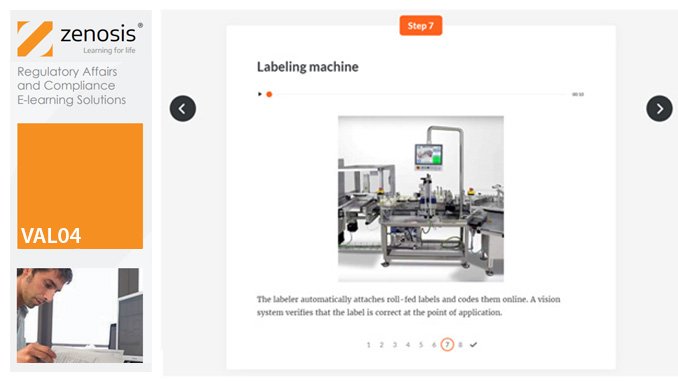
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
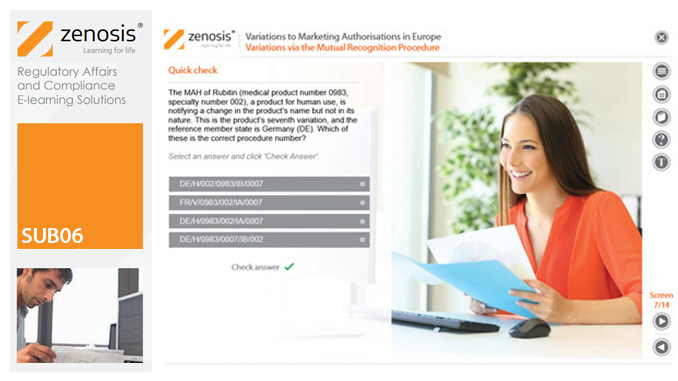
SUB06: Variations to Marketing Authorisations in Europe
By Zenosis
Changes to the terms of marketing authorisations for medicinal products, called variations in Europe, must be notified to or approved by the relevant regulatory authorities. Variations include changes to the composition of products, their manufacturing processes, the way they are used, or the indications for which they are authorised. Common approaches are adopted within the European Economic Area to variations to marketing authorisations approved through the Centralised, Decentralised or Mutual Recognition Procedures. Recent legislation has substantially modified the regulatory requirements and extended them to purely national authorisations by member states. This module, which is fully up to date with the new legislation, covers the classification of variations into their several types and the regulatory requirements, guidance and procedures to be followed for each type.
ESS01: Essentials of EU and US Regulatory Affairs for Human Medicinal Products
By Zenosis
This foundation-level module is the ideal introduction for new entrants to the field of pharmaceutical regulatory affairs and compliance. It describes the principal requirements that must be satisfied to gain and maintain approval to market medicinal products in the USA and Europe. The legal framework and the roles of major players in regulation are presented. The life-cycle of a drug is outlined. The various procedures available for assessment and approval of products are described and their requirements outlined. Obligations to be fulfilled after marketing approval are discussed.

Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant
4.7(47)By Academy for Health and Fitness
Feeling stuck in your career? Struggling to keep up with the ever-changing demands of the industry? You're not alone. But there's good news: Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant career bundle is here to equip you with the essential skills and knowledge you need to break free and achieve your goals. With this Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant course you will get 20 CPD Accredited PDF Certificates, Hard Copy Certificate of Health and Social Care Certificate and our exclusive student ID card absolutely free. Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant premium bundle consists of 20precisely chosen courses on a wide range of topics essential for anyone looking to excel in this field. Each segment of Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant is meticulously designed to maximise learning and engagement, blending interactive content and audio-visual modules for a truly immersive experience. Embarking on Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant is more than just taking an online course; it's an investment in your future. By completing this Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant bundle, you'll not only gain invaluable skills but also open doors to new career opportunities and advancements, boosting your earning potential. Don't miss this chance to elevate your career and skillset. Enrol in Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant today and take the first step towards achieving your goals and dreams. Why buy this Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant? Free CPD Accredited Certificate upon completion of Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant Get a free student ID card with Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant Lifetime access to the Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant course materials Get instant access to this Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant course Learn Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant from anywhere in the world 24/7 tutor support with the Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant course. Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant is an entirely online, interactive lesson with voiceover audio Start your learning journey straightaway with our Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant Training! Courses Are Included In This Bundle: Course 01: Health and Social Care Certificate Course 02: Healthcare GDPR Training Course 03: International Healthcare Policy Course 04: UK Health and Care Course 05: Person-centred Practice in Adult Care Course 06: Basic First Aid Course 07: Study Skills for Healthcare Course 08: Observation Skills for Carers Course 09: Care Planning and Record Keeping | Health & Safety Online Course Course 10: Medical Law Course 11: Nutrition and Hydration Course 12: Public Health Course 13: Social Media in Healthcare Course 14: EFT - Emotional Freedom Technique Course 15: Overcoming OCD with Mindfulness & CBT Course 16: Cognitive Behavioural Therapy (CBT) Practitioner Certificate Course Course 17: Physiotherapy Assistant Course 18: Introduction to Midwifery Certificate Course Course 19: Palliative Care Worker Course 20: Risk Assessment in Health & Social Care Certification You have to complete the assignment given at the end of the Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant course and score a minimum of 60% to pass each exam. Our expert trainers will assess your assignment and give you feedback after you submit the assignment. After passing the Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant exam You will be entitled to claim a PDF & Hardcopy certificate accredited by CPD Quality standards completely free. CPD 200 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant course is ideal for: Students seeking mastery in Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant Professionals seeking to enhance Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant skills Individuals looking for a Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant-related career. Anyone passionate about Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant Requirements This Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant doesn't require prior experience and is suitable for diverse learners. Career path This Care Certificate 15 Standards with Health & Social Care Diploma for Healthcare Assistant bundle will allow you to kickstart or take your career in the related sector to the next stage. Certificates CPD Accredited Digital certificate Digital certificate - Included CPD Accredited Hard copy certificate Hard copy certificate - Included If you are an international student, then you have to pay an additional 10 GBP for each certificate as an international delivery charge.

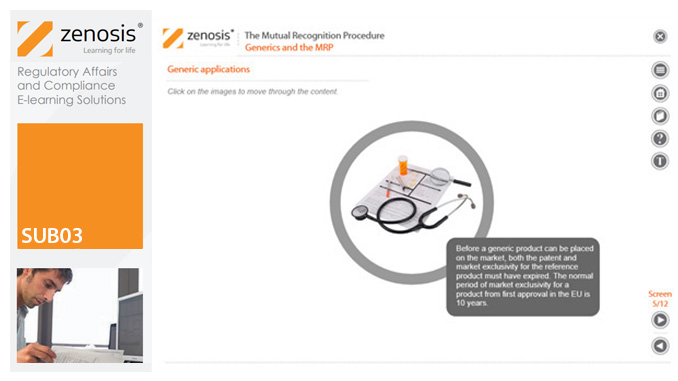
SUB03: The Mutual Recognition Procedure (MRP)
By Zenosis
his module describes the roles of the various players in the procedure, the sequence and duration of the stages involved, and the requirements on content, format and timing of submissions. It discusses the special issues that apply to generic products in the MRP.
CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
By Zenosis
The sponsor of a clinical trial must arrange for it to be monitored throughout its duration to ensure that the rights and wellbeing of subjects are protected, the trial data are accurate, complete and verified from source documents, and the conduct of the trial complies with the study protocol, Good Clinical Practice and regulatory requirements. In this module we describe how a Clinical Research Associate (CRA) monitors an ongoing trial to its conclusion.