- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
65970 Courses
ITIL 4 Strategist: Direct, Plan and Improve: On-Demand
By IIL Europe Ltd
ITIL® 4 Strategist: Direct, Plan and Improve: On-Demand The ITIL® 4 Strategist: Direct, Plan, and Improve course is based on the ITIL® 4 Strategist Direct, Plan, and Improve candidate syllabus from AXELOS. This course is based on the ITIL® 4 Strategist: Direct, Plan and Improve IT exam specifications from AXELOS. With the help of ITIL® 4 concepts and terminology, exercises, and examples included in the course, candidates acquire the relevant knowledge required to pass the certification exam. This course provides the practical skills necessary to create a 'learning and improving' IT organization, with a strong and effective strategic direction. It was designed to provide practitioners with a practical and strategic method for planning and delivering continual improvement with necessary agility. It covers both practical and strategic elements, making it the universal module that is a key component to both ITIL® 4 Managing Professional and ITIL® 4 Strategic Leader streams. What You Will Learn At the end of this course, participants will be able to: Understand the key concepts of direction, planning, improvement Understand the scope of what is to be directed and/or planned and know how to use key principles and methods of direction and planning in that context Understand the role of GRC and know how to integrate the principles and methods into the service value system Understand and know how to use the key principles and methods of continual improvement for all types of improvements Understand and know how to use the key principles and methods of Communication and Organizational Change Management to direction, planning and improvement Understand and know how to use the key principles and methods of measurement and reporting in direction, planning, and improvement Understand and know how to direct, plan, and improve value streams and practices Course Introduction Let's Get to Know Each Other Course Overview ITIL® 4 Certification Scheme Course Learning Objectives Course Components Course Agenda Exercises Case Study: Axle Car Hire Case Study: HandyPerson on Demand Exam Details Core Concepts of DPI Key Terms Covered in the Module Module Learning Objectives Basics of Direction Basics of Planning Basics of Improvement Other Core Elements DPI through Service Value Chain and Guiding Principles Key Terms Covered in the Module Module Learning Objectives DPI of the SVS DPI of Guiding Principles Role of Direction in Strategy Management Key Terms Covered in the Module Introducing Strategy Management Developing Effective Strategies Implementation of Strategies Key Terms Covered in the Module Module Learning Objectives Managing Risks Making Decisions through Portfolio Management Directing via Governance, Risk, and Compliance (GRC) Introduction to Assessment and Planning Key Terms Covered in the Module Module Learning Objectives Core Concepts of Assessment Conducting Effective Assessments Core Concepts of Planning Assessment and Planning through VSM Key Terms Covered in the Module Module Learning Objectives Introducing VSM Developing Value Stream Maps Knowing More About VSM Measurement, Reporting, and Continual Improvement Key Terms Covered in the Module Module Learning Objectives Measurement and Reporting Alignment of Measurements and Metrics Success Factors and Key Performance Indicators Continual Improvement Measurements and Continual Improvement through Dimensions and SVS Key Terms Covered in the Module Module Learning Objectives Measurements for the Four Dimensions Continual Improvement of the Service Value Chain and Practices OCM Principles and Methods Key Terms Covered in the Module Module Learning Objectives Basics of OCM OCM throughout DPI and Service Value Chain Resistance and Reinforcement Communication Principles and Methods Key Terms Covered in the Module Module Learning Objectives Basics of Effective Communication Communication with Stakeholders SVS Development Using Four Dimensions Key Terms Covered in the Module Module Learning Objectives Organizations and People in the SVS Partners and Suppliers in the SVS Value Streams and Processes in the SVS Information and Technology in the SVS

Strategic Thinking and Decision Making: On-Demand
By IIL Europe Ltd
Strategic Thinking and Decision Making: On-Demand The goal of this course is to provide you with the building blocks and the motivation to develop the critical skill of strategic thinking. The participants will consider a four-part model that distinguishes strategic thinking from strategic planning and managing. With that understanding, you will investigate the critical components of strategic thinking and how to apply it effectively. What You Will Learn At the end of this program, you will be able to: Define strategic thinking and distinguish it from strategic planning and management Explain a high-level approach to gaining strategic thinking skills Integrate other interpersonal skills, such as self-awareness, systems thinking, leadership, constructive conflict, and collaboration, into the fabric of strategic thinking skills Select appropriate techniques to apply strategic thinking in specific situations Recognize and emulate effective strategic thinking behaviors Foundation Concepts Strategic thinking versus strategic planning, decision making and management Strategic thinking attributes Strategic Thinking Critical Success Factors Strategic thinking competency and tools at an organization level Strategic Thinking Tools and Techniques at an Individual Level Interpersonal and Team Emotional Intelligence Team Leadership and Trust Constructive Conflict and Collaboration Applying the Critical Skill of Strategic Thinking Team versus client Trusted Advisor

Project Leadership Skills - Francais (On-Demand)
By IIL Europe Ltd
Project Leadership Skills - Français (On-Demand) Pour être efficace au sein d'une organisation, les responsables de projet doivent avoir une grande variété de compétences et d'aptitudes. Parmi celles-ci figurent: la création et l'exécution d'une vision; motiver les autres; influencer sans autorité; la mise en réseau; communiquer haut, bas et latéralement; la négociation; gestion des parties prenantes; et gérer les conflits. Cet atelier vise à développer les compétences générales essentielles à la direction d'une équipe et à la création d'un changement d'entreprise durable. Les participants auront un aperçu des sciences sociales et de la science du cerveau pour motiver et responsabiliser les autres. Ils apprendront et expérimenteront diverses stratégies et tactiques d'influence. Les participants découvriront également leurs préférences personnelles en matière de communication, leurs points forts et leurs angles morts et apprendront comment communiquer au mieux avec d'autres personnes qu'ils jugent «difficiles». Ils apprendront mieux à gérer le côté humain du changement et à apprendre des stratégies pour gérer chaque étape. Dans le processus. Les activités pratiques de négociation et de gestion des conflits renforcent l'apprentissage théorique, l'enracinant dans la vie réelle et le rendant exploitable. What you will Learn At the end of this program, you will be able to: Explain the importance of a vision in driving motivation and engagement Apply a scientific approach to better motivate those around you Strategically maximize your personal and positional power for better project results Determine influence and network development strategies necessary for personal development Know how to respond to communication challenges related to different personality styles Make the link between the expectations of stakeholders and the success criteria of a project Assess key stakeholders across different dimensions of complexity Apply the four rules of principled negotiation to real-life conflict situations Recognize key aspects of a physiological response to conflict Make the right choice of tools and techniques to "demine" an emotional situation Maximize different strategies and tactics to manage ambiguity at work Manage vision and purpose / Motivate others Communication and alignment with the vision Link the present to the future The Importance of Purpose The art and science of motivation Network development and influence Positive policy and project success Types of power in an organization power and influence Network Development Best Practices Communication The medium and the message Personality and communication styles Communication challenges Stakeholder management / Negotiation Identification of stakeholders Stakeholder analysis The basics of negotiation reasoned negotiation Manage the conflict Conflict dynamics The Anatomy of Conflict Conflict management approaches and tools Dealing with Ambiguity

Leading People Through Change (FREE L&I Conference Course)
By IIL Europe Ltd
The goal of this course is for you to effectively lead and manage people through times of change. Research shows that 70% of change initiatives fail in large organizations. The largest factor contributing to this failure rate is leadership - the inability to plan and lead people through change. In many change situations, tremendous focus is put on strategy, processes, and systems, while the issue of changing people's behavior is assumed it will 'just happen'. In this interactive course, you will learn why the people's side of change is crucial. We will begin by understanding why and how people resist change, and how important it is to become strong and effective change champions. Next, we will focus on critical change management practices - creating our vision of the future state, planning for acceptance in our change audience and stakeholders, mitigating threats, and capitalizing on opportunities. We will use metrics to plan, show progress, and confirm success. Lastly, we will focus on the need to reinforce and sustain change, and to prevent relapse to old ways and methods.

Building High Performance Project Teams
By IIL Europe Ltd
This course pulls together the most current and popular theories and writings on this complex topic and presents this amalgamated view in a highly interactive workshop and activity-based approach. Students will understand and have the skills required to build and participate in high-performance project teams and will possess the insight to proactively affect change within their respective organizations by guiding the existing culture to one that promotes high performance.

Management of Portfolios (MoP) Foundations: On-Demand
By IIL Europe Ltd
Management of Portfolios (MoP®) Foundations: On-Demand The purpose of the Foundation certification is to confirm that you have sufficient knowledge and understanding to work as an informed member of a Portfolio Office or in a range of portfolio management roles. In this MoP Foundation course, participants will acquire the sufficient knowledge and understanding of the principles, cycles, practices, techniques, roles, responsibilities, documents, and organizational context within which portfolio management operates. MoP helps organizations ensure if the investments are done in the right change initiatives and implementing them correctly. This is achieved by: Prioritizing the programs and projects in terms of their contribution to the organization's strategic objectives and overall level of risk Managing the programs and projects consistently to ensure efficient and effective delivery Maximizing the benefit by providing the greatest return from the investment made What You Will Learn Individuals certified at the MoP Foundation level will be able to: Define the scope and objectives of portfolio management and how it differs from program and project management List the benefits of applying portfolio management Explain the context it operates in List the principles upon which successful portfolio management is based on List the different approaches to implement MoP List the factors required to maintain the progress and assess the success of portfolio management State the purpose and key content of the major portfolio documents Define the scope of key portfolio management roles Introduction: MoP Scenario Background of the MethodologyThe MoP Principles Senior Management Commitment Governance Alignment Strategy Alignment Portfolio Office Energized Change Culture The MoP Definition CycleRoles and ResponsibilitiesThe MoP Delivery Cycle Management control Benefits management Financial management Risk management Stakeholder engagement Organizational governance Resource management The MoP Framework

Managing and Leading Projects Across Organizational Boundaries: On-Demand
By IIL Europe Ltd
Managing and Leading Projects Across Organizational Boundaries - Achieving Project Success in Complex Environments Through Collaborative Skills: On-Demand Significant projects today are performed by teams of people from multiple organization units and, often, multiple companies. When project managers, team leaders or technical professionals seek collaborative relationships across organizational boundaries, they often encounter a daunting array of challenges. These challenges must be dealt with effectively across business, political, team, interpersonal and personal levels to successfully meet project objectives. The goal of this course is to provide participants with a framework for improving project performance by successfully navigating through the turbulence of organizational cultures. What You Will Learn You'll learn how to: Assess an organizational culture's challenges and adapt your interpersonal skills and political acumen to meet them Apply basic tools and techniques for building relationships and gaining influence across organizational boundaries Plan a tailored, systematic approach for gaining support, resources and collaboration from individuals in organizations where you have no formal influence Getting Started Introductions Course structure Course goals and objectives Foundation Concepts Overview of key course concepts Managing and leading: the balance and evolution Managing and leading projects versus ongoing work Organizations defined and a project manager's outlook across the structures The Path to Competency in Managing and Leading Projects Across Organizational Boundaries (MLPAOB) Organizational Cultures and Behaviors Overview of organizational culture and behaviors Organizations and change Organizational grassroots changes Political Acumen Overview of role and impact of political acumen Role of politics in organizations Political behaviors How to improve your political acumen skills Building Relationships Strategies for building relationships Balance emotion with reason "Try to understand" Inquire, consult and listen Reliability and building trust Gaining Influence Overview of gaining influence Step 1: Determine influence needs Step 2: Assess influence assets Step 3: Plan approach Step 4: Implement plan Step 5: Manage progress Planning and Implementing Your Approach Overview of Implementation Approach and Continuous Improvement (IA&CI) Enhancing Primary MLPAOB skills through: Identifying and sampling auxiliary MLPAO skills Practicing MLPAOB skills Self-reflection: developing a personal action plan (optional - time permitting) Summary What did we learn, and how can we implement this in our work environments?

Women in Healthcare Leadership Workshop Our Aims For This Workshop: Become clear about your leadership style and philosophy. Understanding how to navigate yourself and your team in a VUCA environment. How to communicate with presence and impact. Topic 1 Foundations For Effective Leadership Develop your leadership story – (know yourself) Your values, influences and leadership philosophy Clear vision and purpose as a leader Adapting style to suit the context Topic 2 Leading Through Rapid Change (Uncertainty) Exploring the VUCA environment (Volatile, Uncertain, Complex, Ambiguous) Understanding the psychological process of change Resilience and stress management Topic 3 Communication Skills For Influential Leaders How to prepare to be present so you have a presence Explore and understand your own innate communication style Importance of voice, pace, trust, and rapport THE FACILITATOR Ruth Sangale Ruth has 20 years HR and OD experience in the public and private sectors, leaving the NHS in 2012 to set up her own business “Enjoy Work” and specialise in Creative Leadership development and executive coaching. She works internationally coaching and running residential leadership programs for global organisations such as UNICEF, WHO, UN Women, and PLAN International and charitable NGOs in a range of countries including, Afghanistan, Brazil, India, Kenya, and Belize. In the UK she designs and delivers workshops for mostly the NHS, on topics such as career development, resilience and positive psychology, feedback skills, coaching skills for leaders and team development. She is an ICF-accredited coach and has an M Sc in Innovation, Creativity and Leadership. In her work she uses creative tools such as drama, visualization, drawing, mindfulness and storytelling to stimulate creative thinking and develop leadership capability. She has two daughters and in her free time loves hiking, climbing mountains and salsa dancing. THE PANEL Sam Foster - Chief Nursing Officer - Oxford University Teaching Hospitals Sam joined the Board of Oxford University Hospitals NHSFT in September 2017 as an experienced Chief Nurse who previously worked at the Heart of England NHS Foundation Trust where she held the role of Chief Nurse for four years. Sam has also worked in a number of Trusts in clinical, operational and educational roles. Sam's portfolio includes the professional leadership and education of over 5,000 Nurses, Midwives and AHPs. In addition to the Executive leadership of the Trust Facilities and PFI Services, she is accountable for the Estates and delivery of the capital program. Sam leads the Urgent Care program across the Oxfordshire system. Avey Bhatia - Chief Nursing Officer - Guys & St Thomas’ NHS Trust Avey Bhatia is Chief Nurse at Guy’s and St Thomas’ Trust. Avey returned to the Trust as Chief Nurse in November 2020, having trained as a critical care nurse at St Thomas’ in the early part of her career. Avey qualified in 1991 and her clinical experience includes theatres, general intensive care, coronary care and cardiothoracic nursing. She held various staff nurse and sister posts at hospitals in London before becoming Chief Nurse and Director of Infection Prevention and Control at St George's University Hospitals NHS Foundation Trust in 2017. Avey holds a postgraduate diploma in health services management and a Masters in Public Administration. She is also the Trust’s Director of Patient Experience, and the executive lead for adults’ and children’s safeguarding, and for infection, prevention and control. Beyond Guy’s and St Thomas’, Avey is Vice President for the Florence Nightingale Foundation and Honorary Vice President of The Nightingale Fellowship. She is a Trustee for the St John of Jerusalem Eye Hospital Group. Caroline Alexander CBE - Group Chief Nurse - Barts NHS Trust Caroline graduated as a nurse in 1987 from Edinburgh University (BSc/RGN) and has an MSc in Nursing Studies from South Bank University (2001). From 1987 to 1993 she specialised in nursing older people in Edinburgh and then London at Guy’s Hospital as a ward sister. Caroline then worked for the Foundation of Nursing Studies for three years supporting nurses to use research in practice. In 1998 Caroline returned to the NHS and worked in Tower Hamlets in a range of roles within older people’s services. In 2005, Caroline took up her first Director post, as Director of Nursing and Therapies within Tower Hamlets PCT. With the clustering of PCTs in London in 2011, she took on the Director of Nursing and Quality within NHS East London and the City initially and then within NHS North East London when the clusters merged in 2012. until she joined NHS England as Regional Chief Nurse for London in April 2013. Caroline took up her current role of Chief Nurse for Barts Health in March 2016. Caroline was a 2008 Florence Nightingale Leadership Scholar and has been awarded Honorary Doctorates from City, University of London in 2017, Middlesex University in 2018 and University of East London in 2021. She is a Trustee of the Foundation of Nursing Studies. In 2020 she was made a CBE in the Queen’s Birthday Honours. Who will attend? Emerging Leaders looking to step into management roles Current Leaders looking to progress into senior management roles This workshop is open to any woman who works in health care and wants to take her next step in their career; women include trans women and non-binary people who are comfortable in a female-centered group. Group Rate Discounts 2-3 people, 7% discount 4+ people, 20% discount We have two group rates which you can take advantage of depending on the size of the group you wish to book: Option 1️⃣ Groups between 2 & 3 are eligible for the 7% Discount. Please use this code at checkout: GROUP 2+ Option 2️⃣ For groups of over 4+ attendees, the eligible discount is 20%. Please use this code at checkout: GROUP 4+ Where do I add the discount code?

Microneedling Training
By Cosmetic College
We have completed a training course to teach you everything you need to know about Microneedling. Microneedling, also known as skin needling or collagen induction therapy, leads the way in skin rejuvenation. Safely delivered, this treatment carries no risk or downtime to the client. It is a procedure whereby a device, such as a derma roller or an automated pen device, is passed across the skin's surface, creating superficial punctures that stimulate a healing response. We work with Dr Pen and Beautier needling pen and the Clinicare range as part of this course and cover appropriate products from this range. Skills and knowledge from this course will be transferable to other brands. Our Microneedling Training Course will provide you with everything you need to know about the history and the use of microneedling, as well as all the technical aspects of it: safety, indications, technique and post-treatment tips for a successful outcome. This course will primarily focus on the face, although we touch on the scalp & body benefits of this treatment. Learn how to safely, confidently and expertly deliver this treatment with our fully accredited microneedling training course Course prerequisites Minimum 18 years of age Good command of English Be able to learn independently A strong desire to build a career in aesthetics Previous skin and facial training are desirable; we suggest that learners new to the industry enrol on our facial and skincare course before enrolling on our ClinicCare skin peel course. Course structure The online study, virtual lecture and in-person practical training All courses are intimate with four learners in class 2-1 ratio. One day of on-site training Online learning includes: Anatomy and physiology of the skin and tissues Infection control Sharps and hazardous waste training History of skin needling Treatable skin conditions Contraindications Consultation Aftercare Introduction to automated Pen Device and Dermaroller Pre-study microneedling (health and safety) Onsite training day includes: Face-to-face practical training with 1 model per treatment area Clinical set up Live treatment demonstrations Frequently Asked Questions Where is the Cosmetic College The Cosmetic College is located at: 3 Locks Court, 429 Crofton Road, Orpington, BR6 8NL How can I book? We have a few options for you to book. You can book by selecting an available training date above here on our website, by contacting us through email at hello@cosmetic.college or by contacting us on 0333 015 5117. Is a deposit required to book? All enrolments are charged an administration fee which is non-refundable. When you enrol you can elect to pay a deposit of 10% plus the administration fee or pay the total training course in full. We have full details of the terms and conditions of training course enrolments here What is the course duration? 1 day + pre-study via our online learning platform.
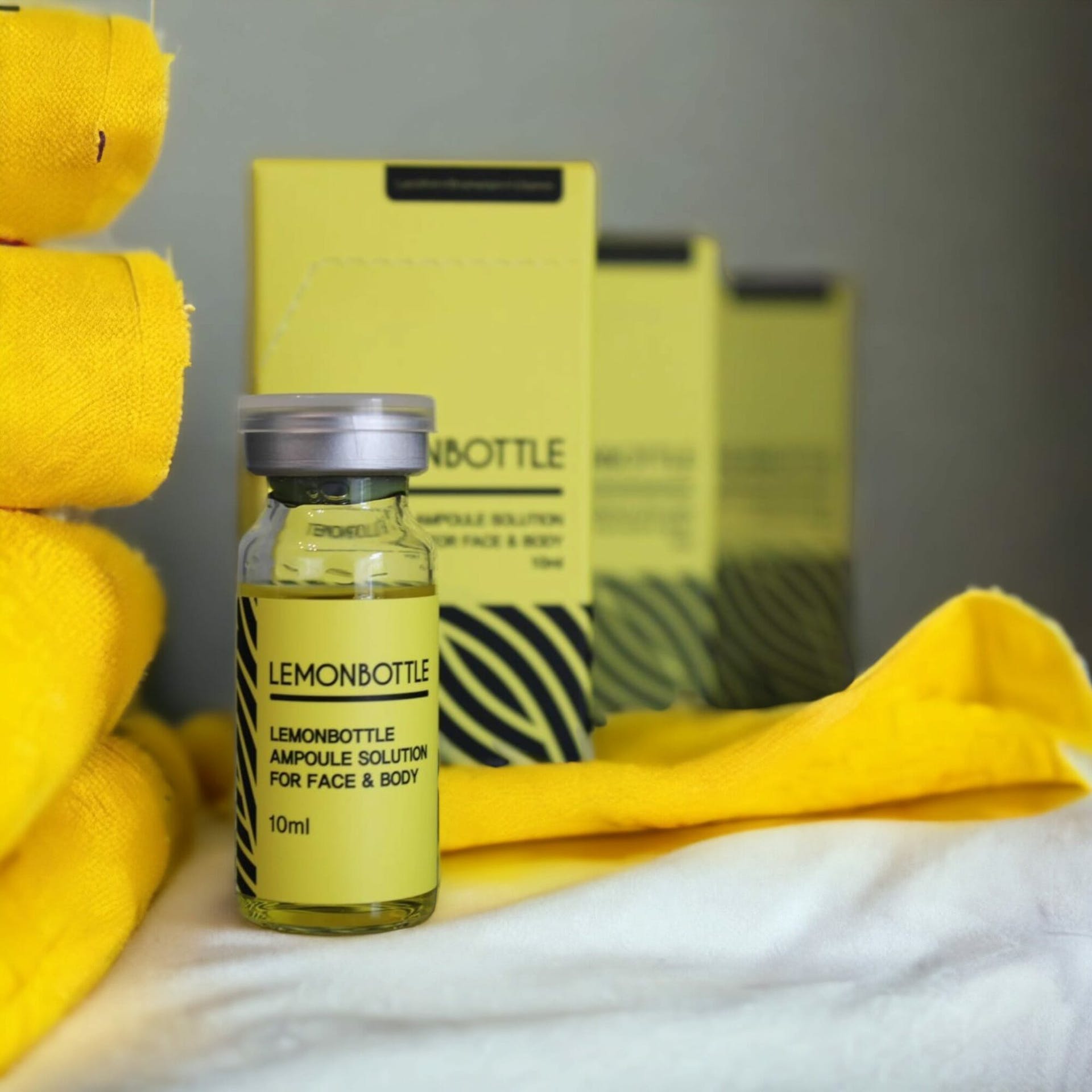
Lemon Bottle Fat Dissolving Training
By Cosmetic College
Lemon Bottle can be used on the face and body to great effect. Just one session can dramatically reduce fat deposits and return a more youthful and defined facial contour for small areas such as the jowls and chin. For larger areas like tummies, bingo wings and bums, clients may need up to 5 sessions to see dramatic results. Lemon Bottle are water-based solutions injected into the fatty tissue, surround the cells and destroy them. The remains of the fat cells are then excreted by the body safely as waste. Lemon Bottle is used for people who want to get rid of stubborn areas of fat in their faces and bodies and is clinically proven for these purposes. Course Entry Requirements: This course is suitable for both medics and non-medics. You can enrol on this training course if you meet one of the following: NVQ Level 3 in beauty therapy, ITEC or HND Medically qualified as a nurse, doctor or dentist with current registration with the NMC, GMC or GDC. 12 Months of Needling Experience or 6 Months of Needling Experience and Anatomy & Physiology Level 3 or Above. Course Structure: 20 hours of pre-study E-learning and 1 practical training Course Agenda: Areas covered within the course: Anatomy and physiology of the skin and tissues Infection control Sharps and hazardous waste training First aid and anaphylaxis training Pre-study fat-dissolving theory training Practical training Clinical set up Professional live demonstrations Frequently Asked Questions Is this course suitable for beginners or those with prior experience? Lemon Bottle Fat Dissolving Training Course is designed for aesthetics practitioners who already have a foundation in basic injection techniques and experience in the field. Prior experience in administering injections is essential to enrol in this advanced course. Will I receive a certification upon completion? Yes, upon successfully completing our training course, you will receive a certification in Lemon Bottle Fat Dissolving. This certification acknowledges your advanced skills and expertise in administering these fat dissolving treatments. It can enhance your professional reputation and provide a competitive edge in the aesthetics industry. What topics are covered in the course curriculum? Our course curriculum covers various topics related to Lemon Bottle fat dissolving treatments. These include product knowledge, patient selection and assessment, injection techniques, treatment protocols, managing complications, and post-treatment care. The curriculum is designed to provide you with a comprehensive understanding of the Will I have access to ongoing support after completing the course? Absolutely! We provide ongoing support to our students even after they complete the training course. Our instructors and support staff are available to answer any questions, provide guidance, and offer assistance as you navigate your career in Aqualyx and Deso fat dissolving treatments. We are committed to supporting your continued growth and success. Are there financing options available for the course? We offer flexible payment options and financing plans to make our Lemon Bottle Fat Dissolving Training Course more accessible. Please reach out to our admissions team for detailed information on available payment options and financing plans.
Search By Location
- Courses in London
- Courses in Birmingham
- Courses in Glasgow
- Courses in Liverpool
- Courses in Bristol
- Courses in Manchester
- Courses in Sheffield
- Courses in Leeds
- Courses in Edinburgh
- Courses in Leicester
- Courses in Coventry
- Courses in Bradford
- Courses in Cardiff
- Courses in Belfast
- Courses in Nottingham