- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
65656 Courses
Back to Unschool
By LivePlayLearn
what does it cost to unschool?

Couples Therapy: Beyond Foundations - Full Recording
By Practical CBT
Full recording of the workshop: Couples Therapy - Beyond Foundations You will have access to the recording for 14 days.

Corporate Finance & Investment
By Eduolc
This course will teach you the fundamentals of financial statement and decision analysis, with a focus on the balance sheet. One of the most basic qualities in the corporate world is the ability to assess a company's results.

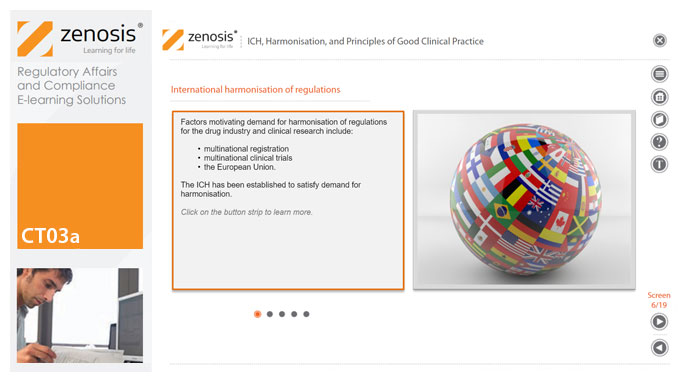
CT03a - ICH, harmonisation, and principles of Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data in applications for marketing approval. The International Council for Harmonisation's guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice. In this short course we describe the ICH’s role in the harmonisation of regulations, introduce its guideline E6, and set out the principles of GCP.
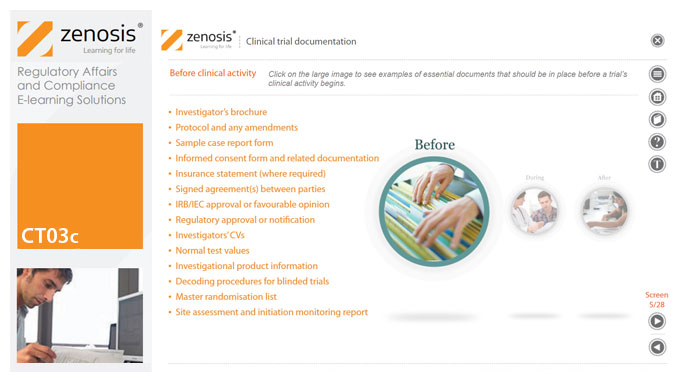
CT03c - Clinical trial documentation
By Zenosis
Regulatory authorities tend to abide by the maxim that ‘If it isn’t documented, it didn’t happen’. Rigorous documentation of all aspects of a clinical trial is necessary to provide evidence of GCP and compliance with regulatory requirements, as well as enabling effective management of the trial. In this short course we describe important examples of the documents designated by ICH GCP as essential to the conduct of a clinical trial.
Treating GAD - Full Recording
By Practical CBT
Get 28 days access to the video recording of 'Treating GAD' with Professor Patrick McGhee, FRSA

Trusting Your Child - In A World That says That Children Cannot be Trusted
By LivePlayLearn
This webinar explores the depths of the revolutionary idea of trusting your child, rumoured to be the hardest aspect of unschooling, but also the change that could dramatically improve the dynamics in your home and your child's learning capabilities.

Treating Depression - Full Recording
By Practical CBT
Get 28 days access to the video recording of 'Treating Depression' with Professor Patrick McGhee, FRSA

Report Writing for Unschoolers
By LivePlayLearn
Life is full of learning and as unschoolers your children are learning through their own lives. But now it comes to conversations with your Local Authority and their need to be assured that your child is in receipt of a suitable education, how do you translate your child's day to day activities into an educational report?

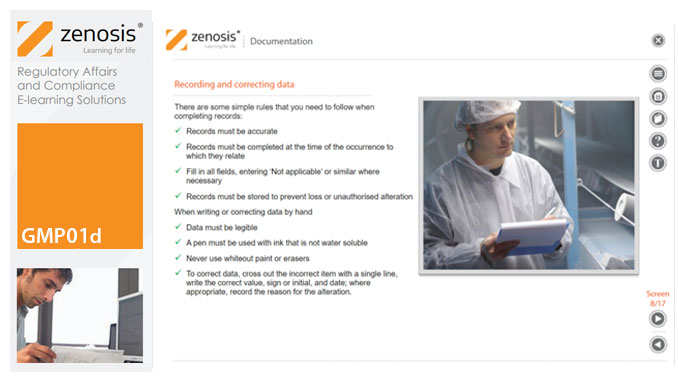
GMP01d - Documentation
By Zenosis
Comprehensive documentation of procedures, formulas, work instructions, and specifications, and thorough recording of batch data, are fundamental requirements of GMP. In this short course we explain why documentation is so important, identify different types of document required, and set out some simple rules for recording and correcting data.
Search By Location
- Courses in London
- Courses in Birmingham
- Courses in Glasgow
- Courses in Liverpool
- Courses in Bristol
- Courses in Manchester
- Courses in Sheffield
- Courses in Leeds
- Courses in Edinburgh
- Courses in Leicester
- Courses in Coventry
- Courses in Bradford
- Courses in Cardiff
- Courses in Belfast
- Courses in Nottingham