- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
59737 Courses
Open to any BBUK Leaders who have fully completed the registration process. Safeguarding and Managing Risk e-learning modules must be completed prior to attending the training course. Saturday 2nd November 2024 9:45 am to 5:45pm covers Modules 1: Introduction to BB, Module 6: Equal Opportunities, Module 2: Working with Under 11's OR Module 3: Working with Over 11's, Module 4: Safeguarding (recognised by the Church of Scotland) Module 4 will start at 3:15pm and can be attended by Leaders who need this for Church of Scotland congregational purposes. Sunday 3rd November 2024 1:45pm to 7pm covers Module 5: Managing Risk, Module 7: Programme Planning & Delivery & Module 8: Sharing Faith

If you're an intermediate silver clay artist and want to take your skills to the next level, Advanced Silver Jewellery Workshop is for you. In this workshop, you'll learn how to make rings, filigree, and mirror-polished pieces with advanced techniques. You'll leave with a set of quality products that will show your true talent as an artist. And best of all? You'll be able to use these skills on more complex projects later on!

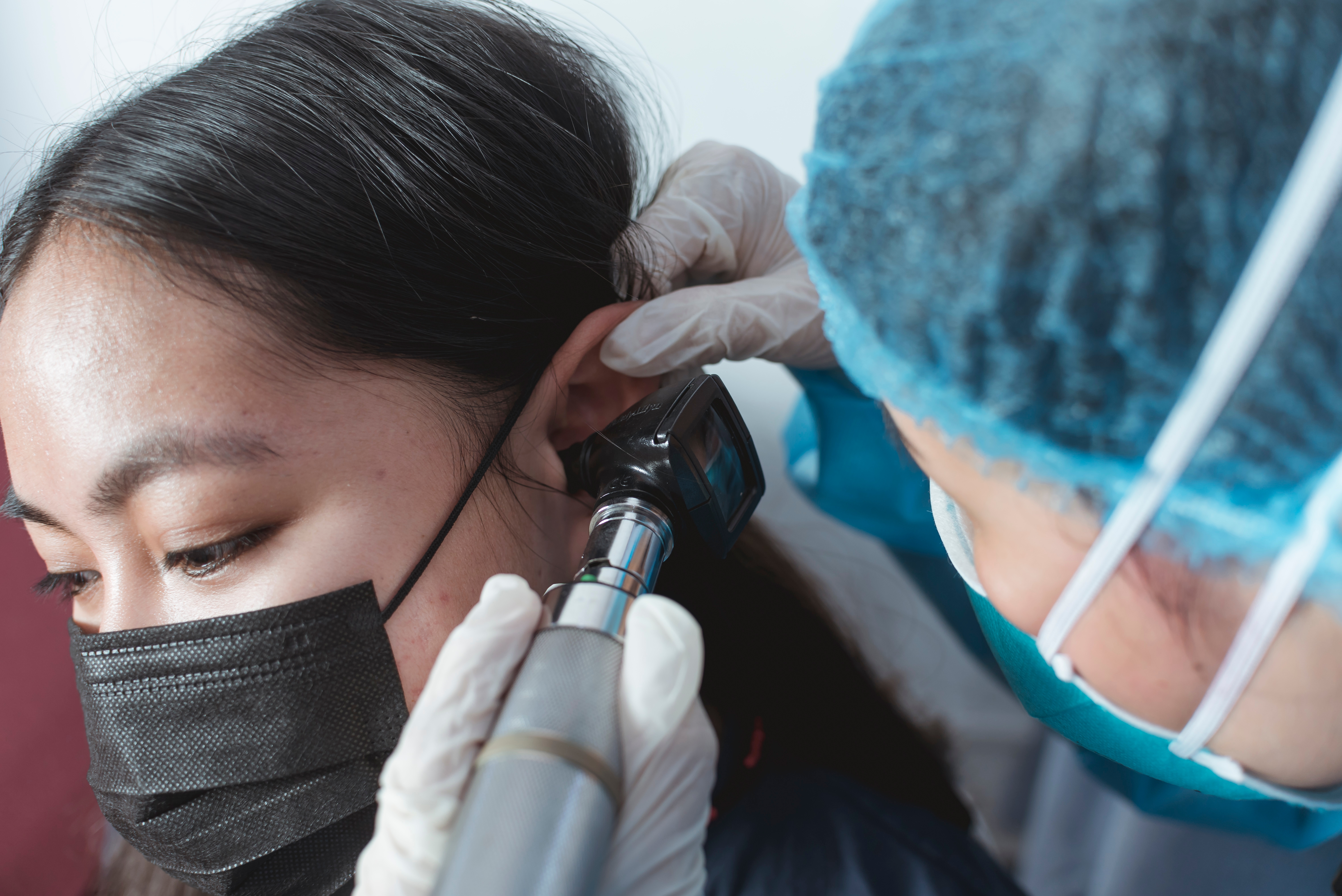
An Understanding of Ear Anatomy and Otoscopy
By Guardian Angels Training
Gain comprehensive knowledge of ear anatomy and otoscopy with our course. Ideal for healthcare professionals, medical students, and audiologists.
Level 5 Diploma in Mental Health Care (QLS Endorsed)
By Kingston Open College
QLS Endorsed + CPD QS Accredited - Dual Certification | Instant Access | 24/7 Tutor Support | All-Inclusive Cost

An Understanding of Pressure Area Care
By Guardian Angels Training
Enhance your knowledge and skills in pressure area care with our comprehensive course. Ideal for healthcare professionals, caregivers, and nurses.

Quality Assurance for Good Laboratory Practice
By Research Quality Association
Course Information A must-have programme for Quality Assurance auditors stepping into or honing their role within a Good Laboratory Practice (GLP) environment, this course offers invaluable, expert guidance for crafting a robust and efficient GLP audit programme. What will I learn? A solid regulatory foundation underpinning quality assurance activities Clarity on the roles of Quality Assurance, management, and study director within the framework of Good Laboratory Practice principles Enhanced efficacy in inspections and audits Heightened compliance with Good Laboratory Practice standards for your facility Unique insights into governmental monitoring activities within the GLP sphere. This course is structured to encourage delegates to Discuss and develop ideas Solve specific problems Examine particular aspects of GLP. Tutors Tutors will be comprised of (click the photos for biographies): Cate Ovington Director, The Knowlogy Group Ltd Jane Elliston Senior Quality Assurance Auditor, Battelle UK Shona Ross Head of QA, Tower Mains Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Welcome and Introductions 09:15 Good Laboratory Practice Standards and Regulations An insight into the background and history of Good Laboratory Practice. 09:45 Principles of Quality Assurance What is the role and responsibilities of QA in GLP. Maintaining the independence of QA and what is an audit. 10:30 Break 10:45 Standard Operating Procedures GLP requirements and QA involvement. 11:30 Study Plans GLP requirements and QA involvement. 12:05 QA Programme Risk based programme, what are study, process and facility audits. 13:00 Lunch 14:00 Inspections Attitudes, techniques and attributes. 14:40 Workshop 1 - Facility and Process Inspections An exercise in inspection planning and preparation for inspections. 15:15 Break 15:30 Workshop 1 - Feedback 15:45 The Auditor and Audit Conduct Attitudes, attributes and techniques. 16:30 Panel Session An opportunity for delegates to put questions to the panel of speakers. 17:15 Close of Day Day 2 09:00 Workshop 2 - A Mock Audit 10:45 Break 11:00 Workshop 2 - Feedback 11:30 Auditing the Study Report Techniques and methods for the QA audit of the study report. 12:00 Record Keeping and Data The impact of GLP on data and records management. 12:40 Lunch 13:25 Data Integrity A look at the OECD GLP guidance document; the expectations of the regulators and the involvement of QA - Where QA adds value. 14:15 Workshop 3 - Amendments to Study Plan and Deviations from the Plan What are they? What is the difference between them? How are they controlled? 15:00 Workshop 3 - Feedback 15:15 Break 15:30 Regulatory Compliance GLP Monitoring Authority monitoring for compliance with Good Laboratory Practice. 16:15 Panel Session An opportunity for delegates to put questions to the panel of speakers. 16:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Learn

Canva Training Course
By iStudy UK
Want to be a graphic designer? Enrol the Canva Training Course and starts your journey in the amazing graphical world. Canva is a graphic design tool that offers a drag-and-drop format and allows you to access to over a million photographs, graphics, and font. Using the software, you can create an amazing Business card, Presentation card, Images, YouTube Channel Banner, Facebook Page Cover, YouTube Thumbnail, Instagram Post, Desktop Wallpaper, Facebook Ad, Linkedin Banner, eBook, Kindle Cover, Logo, Food & Drink Menu, and many other graphical images. In the course, you will explore how to use the software from creating a wonderful graphical presentation with the simple drag and drop features of the software. The course starts by familiarizing you with the software including its paid and free version and the features and advantages of these two versions. Then the course introduces you with the user interface of the program and helps you to start and make your first images. The strategies for adding backgrounds, uploading new videos, resizing, and navigating the UI of Canva will be discussed in the course. Finally, the course shows you various platforms for selling your images. What you'll learn You'll know how to use Canva You'll know how to navigate the UI of Canva You'll know how to add backgrounds, upload new photos, resize anything, undo/redo and so much more You'll know how to sell your images and make money with your new talent You'll know how to choose the best platform to sell your graphics on Requirements You should know how to use a PC at a beginner level You will need an account at Canva (FREE or PAID) to create your graphics Who is the target audience? Freelancers Graphic Designers Lifestyle Marketers Online Marketers Anyone who wants to make some extra $$$ online quickly Anyone with a product to sell Anyone who wants to do graphic design but you have ZERO talent (yes, it's that easy) Introduction to Canva FREE 00:02:00 Making Money as a Graphic Designer and Where To Do It 00:10:00 Signup for Canva FREE vs PAID 00:03:00 Canva User Interface Walkthrough 00:10:00 Creating Your First Image in Canva 00:10:00 My Hack for Making Graphics 00:18:00 Elements Inside Canva 00:15:00 Course Certification

Search By Location
- course, Courses in London
- course, Courses in Birmingham
- course, Courses in Glasgow
- course, Courses in Liverpool
- course, Courses in Bristol
- course, Courses in Manchester
- course, Courses in Sheffield
- course, Courses in Leeds
- course, Courses in Edinburgh
- course, Courses in Leicester
- course, Courses in Coventry
- course, Courses in Bradford
- course, Courses in Cardiff
- course, Courses in Belfast
- course, Courses in Nottingham