- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
8158 Courses
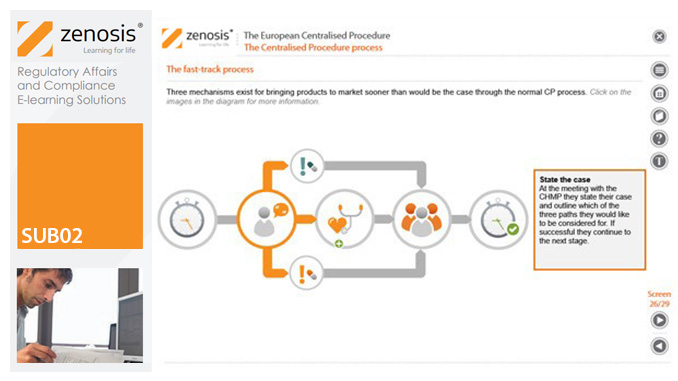
SUB02: The European Centralised Procedure (CP)
By Zenosis
The Centralised Procedure (CP) is one of three routes available to applicants to gain multinational marketing authorisation within the European Economic Area (EEA) on the basis of a single application. In the CP, one successful application leads to a marketing authorisation being issued by the European Commission that applies throughout the EEA. The CP is mandatory for certain types of products.
PKPD01: An Introduction to Pharmacokinetics and Pharmacodynamics in Drug Development and Registration
By Zenosis
Pharmacokinetic (PK) and pharmacodynamic (PD) studies provide a bridge between science and medicine in the development of a drug. In this module we describe the role of in-vivo PK and PD studies in a drug development programme, set out the uses to which the findings can be put, and discuss their implications for clinical development and application for marketing approval.

SUB04: Preparing Submissions in the Common Technical Document (CTD) Format
By Zenosis
The CTD is the internationally recognised standard format for submissions to medicines regulatory authorities. In the European Economic Area, the USA and Canada, the CTD, in its electronic format (eCTD), is mandatory for all applications for marketing approval and all subsequent related submissions. The CTD is accepted in many other countries, being mandatory for new prescription medicines in some. This module explains the rationale for the CTD and provides guidance on its structure and format and the ways in which it is used.

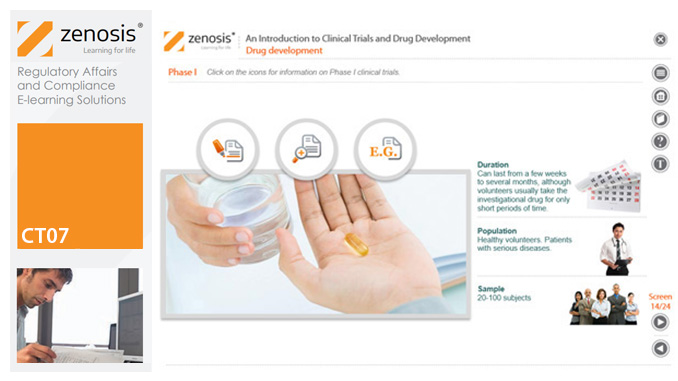
CT07: An Introduction to Clinical Trials and Drug Development
By Zenosis
This module provides an understanding of how clinical trials fit into the drug development process. It outlines the key historical events leading to the development of controlled clinical trials. It specifies the purpose of trials, outlines their features, and identifies codes and regulations that apply to them. Finally, it describes the environment of cost control in which the modern pharmaceutical industry operates.
An Introduction to Gastrostomy Tube Care and Feeding (e-Learning)
By Guardian Angels Training
Learn safe and effective gastrostomy tube care and feeding in our comprehensive e-learning course. Ideal for healthcare professionals, students, caregivers, and medical enthusiasts.

An Introduction to Nasogastric Tube Feeding (e-Learning)
By Guardian Angels Training
Gain essential knowledge and practical skills related to nasogastric tube feeding with our comprehensive e-learning course. Designed for healthcare professionals, students, caregivers, and individuals interested in medical care.

Immediate Management of Anaphylaxis (e-Learning)
By Guardian Angels Training
Gain essential knowledge and practical skills related to anaphylaxis with our comprehensive e-learning course. Designed for healthcare professionals, first responders, students, caregivers, and individuals interested in medical care.
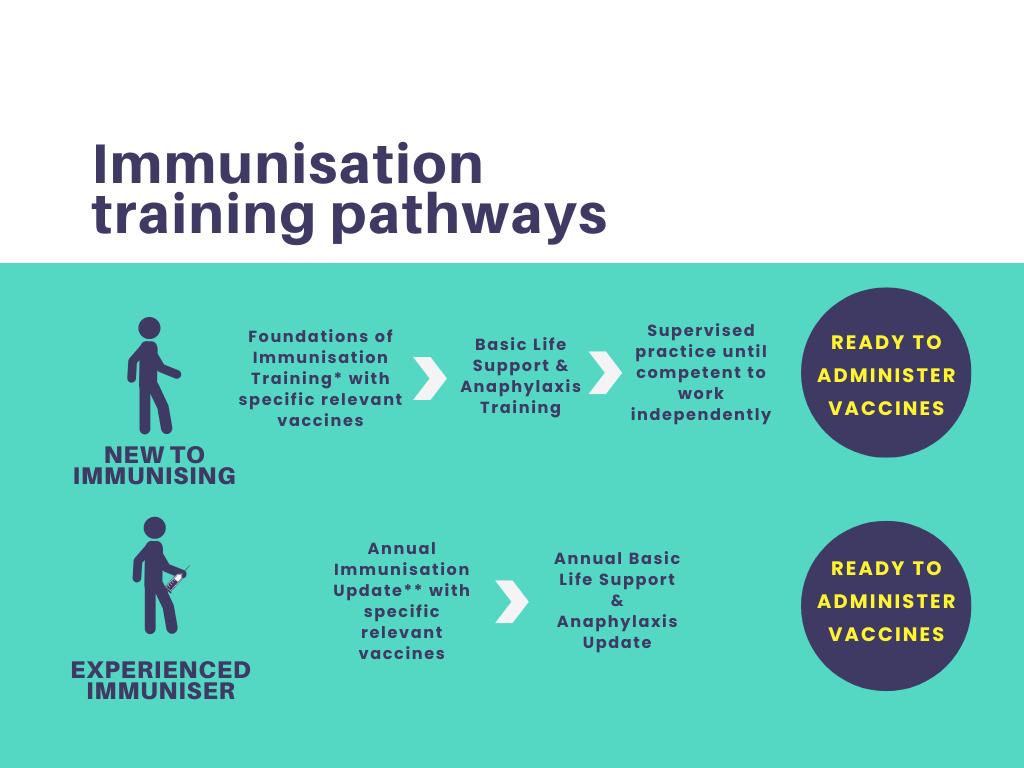
Immunisation and Vaccine (pre-course e-learning)
By Guardian Angels Training
Gain essential knowledge about immunisation and vaccines with our comprehensive "Immunisation and Vaccine Pre-Course e-Learning" program for healthcare professionals, students, caregivers, and public health enthusiasts.
An Introduction Epilepsy and Buccal Midazolam - non-RQF (e-Learning)
By Guardian Angels Training
Gain essential knowledge and skills related to epilepsy management and buccal midazolam administration with our comprehensive e-learning course. Ideal for healthcare professionals, students, caregivers, and individuals interested in epilepsy management.

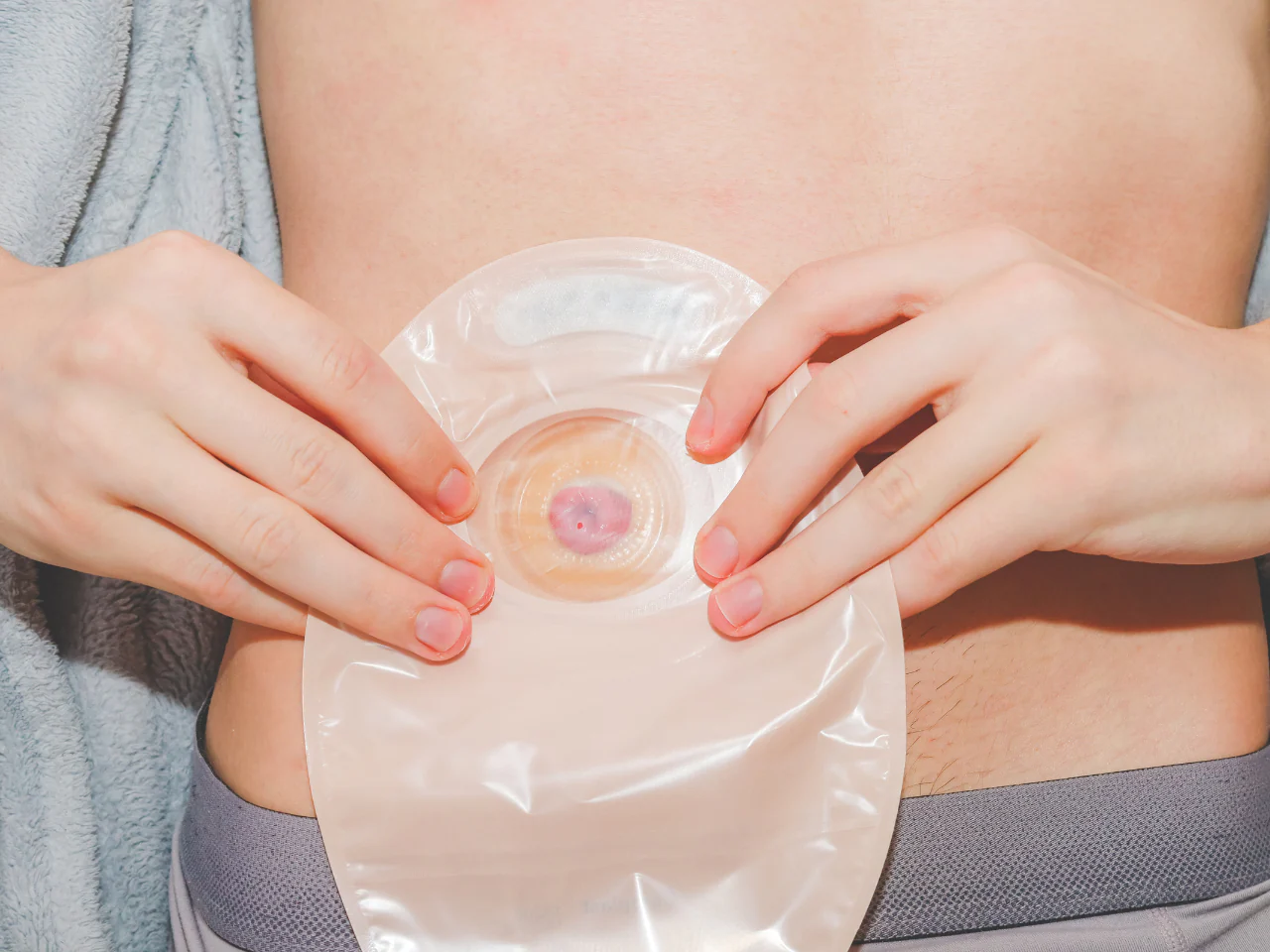
An Introduction to Stoma Care (e-Learning)
By Guardian Angels Training
Gain essential knowledge and practical skills related to stoma care with our comprehensive e-learning course. Designed for healthcare professionals, students, caregivers, and individuals interested in medical care.
Search By Location
- Medicine and Nursing Courses in London
- Medicine and Nursing Courses in Birmingham
- Medicine and Nursing Courses in Glasgow
- Medicine and Nursing Courses in Liverpool
- Medicine and Nursing Courses in Bristol
- Medicine and Nursing Courses in Manchester
- Medicine and Nursing Courses in Sheffield
- Medicine and Nursing Courses in Leeds
- Medicine and Nursing Courses in Edinburgh
- Medicine and Nursing Courses in Leicester
- Medicine and Nursing Courses in Coventry
- Medicine and Nursing Courses in Bradford
- Medicine and Nursing Courses in Cardiff
- Medicine and Nursing Courses in Belfast
- Medicine and Nursing Courses in Nottingham