- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
8158 Courses
Health & Social Care, Medical Law & Public Health - QLS Endorsed
5.0(1)By Academy for Health and Fitness
24-Hour Knowledge Knockdown! Prices Reduced Like Never Before The health and social care sector in the United Kingdom is a vital component of the nation's well-being. According to the Department of Health and Social Care, the NHS employs over 1.3 million people, making it one of the largest employers in the country. However, the sector faces significant challenges, including an aging population, increasing demand for services, and evolving legal and regulatory frameworks. Are you prepared to navigate these complexities and contribute to the delivery of high-quality care? This comprehensive Health & Social Care bundle provides a comprehensive understanding of the health and social care landscape, medical law, and public health practices. You will gain insights into effective management strategies, legal and ethical considerations, and the principles of public health interventions. The courses cover a wide range of topics, including adult nursing, maternity care, care planning, mental health care, and domiciliary care. By completing this bundle, you will be equipped with the knowledge and skills necessary to excel in various healthcare settings. Key Features of the Health & Social Care, Medical Law& Public Health Bundle: 3 QLS-Endorsed Courses: We proudly offer 3 QLS-endorsed courses within our Health & Social Care, Medical Law& Public Health bundle, providing you with industry-recognized qualifications. Plus, you'll receive a free hardcopy certificate for each of these courses. QLS Course 01: Health and Social Care Management QLS Course 02: Medical Law QLS Course 03: Public Health 5 CPD QS Accredited Courses: Additionally, our bundle includes 5 relevant CPD QS accredited courses, ensuring that you stay up-to-date with the latest industry standards and practices. Course 01: Adult Nursing Course 02: Maternity Care Assistant Course Course 03: Care Planning and Record Keeping | Health & Safety Online Course Course 04: Mental Health Care - MCA & DOLS Course 05: Domiciliary Care Support Worker In Addition, you'll get Five Career Boosting Courses absolutely FREE with this Bundle. Course 01: Professional CV Writing Course 02: Job Search Skills Course 03: Self Esteem & Confidence Building Course 04: Professional Diploma in Stress Management Course 05: Complete Communication Skills Master Class Convenient Online Learning: Our Health & Social Care, Medical Law& Public Health courses are accessible online, allowing you to learn at your own pace and from the comfort of your own home. Learning Outcomes of Health & Social Care Evaluate management strategies in health and social care settings. Analyse legal and ethical frameworks relevant to medical practice. Develop strategies for promoting public health and disease prevention. Examine the principles of care planning and record-keeping. Assess mental health care practices and relevant legal frameworks. Integrate best practices in domiciliary and community-based care. Why Choose Us? Get 3 Free QLS Endorsed Certificate upon completion of Health & Social Care Get a free student ID card with Health & Social Care Training program (£10 postal charge will be applicable for international delivery) The Health & Social Care is affordable and simple to understand This course is entirely online, interactive lesson with voiceover audio Get Lifetime access to the Health & Social Care course materials The Health & Social Care comes with 24/7 tutor support Start your learning journey straightaway! *** Course Curriculum *** QLS Course 01: Health and Social Care Management Module 1. Introduction to Health and Social Care Module 2: Communication and its Relevance Module 3: Rights and Responsibilities as a Health and Social Care Worker Module 4: Role as A Caregiver and Healthcare Professional Module 5: Working in Health and Social Care; Promoting Equality, Diversity and Rights Module 6: Important Principles and Policies in Health and Social Care Work Module 7: Understanding Legal, Professional Standards of Practice and Ethical Aspects of Health Care Part - 1 Understanding Legal, Professional Standards of Practice and Ethical Aspects of Health Care Part - 2 Module 9: Safeguarding Vulnerable Individuals Module 10: Health and Safety Responsibilities Module 11 : The Economics of Healthcare Module 12: Strategic Marketing for Health and Social Care Module 13: Managing Finance in Health and Social Care Module 14: Managing Service Delivery in Health and Social Care QLS Course 02: Medical Law Module 1- An Introduction To Medical Law Module 2- Legislation On Access To Health, Medical Report, Treatment Module 3- Legislation On Adult Support Module 4- Legislation On Public Health And Health Service (Part 1) Module 5- Legislation On Public Health And Health Service (Part 2) Module 6- Legislation On Public Health And Health Service (Part 3) Module 7- Legislation On Public Health And Health Service (Part 4) Module 8- Legislation On Coronavirus Module 9- Legislation On Mental Health (Part 1) Module 10- Legislation On Mental Health (Part 2) Module 11- Legislation On Abortion Module 12- Other Legislation (Part 1) Module 13- Other Legislation (Part 2) QLS Course 03: Public Health Module 01: Introduction To Public Health Module 02: Principles Of Public Health Module 03: Understanding Epidemiology Module 04: Disease Control Module 05: Understanding Measures Of Disease Frequency Module 06: Maternity And Childbirth Module 07: Environment And Public Health Module 08: Health System And Policy Module 09: Public Health And Ethics =========>>>>> And 10 More Courses <<<<<========= How will I get my Certificate? After successfully completing the course, you will be able to order your QLS Endorsed Certificates and CPD Accredited Certificates as proof of your achievement. PDF Certificate: Free (Previously it was £12.99*11 = £143) QLS Endorsed Hard Copy Certificate: Free (For The 3 QLS Courses: Previously it was £327) CPD 450 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone interested in learning more about the topic is advised to take this bundle. This bundle is ideal for: New healthcare professionals Career changers Care assistants Support workers Requirements You will not need any prior background or expertise to enrol in this bundle. Career path After completing this bundle, you are to start your career or begin the next phase of your career. Healthcare Manager Medical Lawyer Health Economist Health Policy Analyst Healthcare Consultant Certificates CPD Accredited Digital Certificate Digital certificate - Included Upon passing the Course, you need to order a Digital Certificate for each of the courses inside this bundle as proof of your new skills that are accredited by CPD QS for Free. CPD Accredited Hard Copy Certificate Hard copy certificate - Included Please note that International students have to pay an additional £10 as a shipment fee. Diploma in Health and Social Care at QLS Level 5 Hard copy certificate - Included Please note that International students have to pay an additional £10 as a shipment fee. Diploma in Public Health at QLS Level 4 Hard copy certificate - Included Please note that International students have to pay an additional £10 as a shipment fee.

Course Introduction Covers B12 deficiency, pernicious anaemia, diagnosis, treatment and management. It also covers signs and symptoms Please note: this course is for health care professionals and nurses only. About this event Course Introduction This course concentrates on B12 deficiency, symptoms, treatments and management. The course covers B12 deficiency, pernicious anaemia, diagnosis, treatment and management. It also covers signs and symptoms of pernicious anaemia. This course is interactive and we include case studies and discuss issues regarding diagnostic testing. We review inclusion and exclusion criteria and identification of appropriate clients. Delegates will get the opportunity to review practice with hands on practical demonstrations of how to give injections correctly. We will cover administration techniques, where to give the injections and record keeping / documentation. We will discuss role and responsibilities and contraindications and precautions. The delegates will leave this course with an example of an individual protocol of Patient Specific Direction (PSD) and a competence based framework document to be used in practice. This course is very interactive. AIMS AND OBJECTIVES Understand the need for accountability and responsibility in relation to role development Demonstrate an understanding of safe practice Describe the signs and symptoms of pernicious anaemia Describe pernicious anaemia and its impact on patients Fully understand the principles, and practice B12 deficiency and B12 injections Understand the importance of safety issues related to giving injections Understand the law relating to role and function of the HCA and prescribing. Describe why patients require B12 injectionsBe able to correctly identify anatomical sites for injections Demonstrate correct administration techniquesDemonstrate how to correctly dispose of waste Demonstrate correct infection control procedures and use of PPE Describe when patients require referral and understand the importance of referral using correct clinical pathways Demonstrate an understanding of anaphylaxis and emergency procedures Understand the need for correct prescribing procedures Be able to document consultations following your organisations procedures COURSE CONTENTS Role and responsibilities Accountability guidelines and requirements Pernicious anaemia Blood- function B12 Deficiency Risk factors/groups Causes of B12 deficiency Diagnosis and reference ranges, testing Protocols and guidelines Factors affecting B12 diagnosis and treatment Factors affecting absorption B12 injections and common side effects Could it be B12 Deficiency Supplements Side effects and management including ADR’s Anaphylaxis Contraindications and Precautions Correct Administration and techniques including practical session Injection sites Legal Issues including consent Prescribing and Patient Specific Directions What to record Storage Disposal of injections/waste Infection control Needle stick injuries Competence and supervised practice Policies and procedures Facts and Figures Setting up and running a clinic Insurance/indemnity Research/evidence base and resources WHO SHOULD ATTEND? HCAs Nurses Doctors Pharmacists Anyone interested in Vitamin B12 deficiency and pernicious anaemia and those working with clients with B12 deficiency AB Health Group awards CPD points / certificate of attendance for each course. If you would prefer an accredited certificate by our accrediting body Aim Qualifications we can organise this. The charge for the certificate including postage is £30.
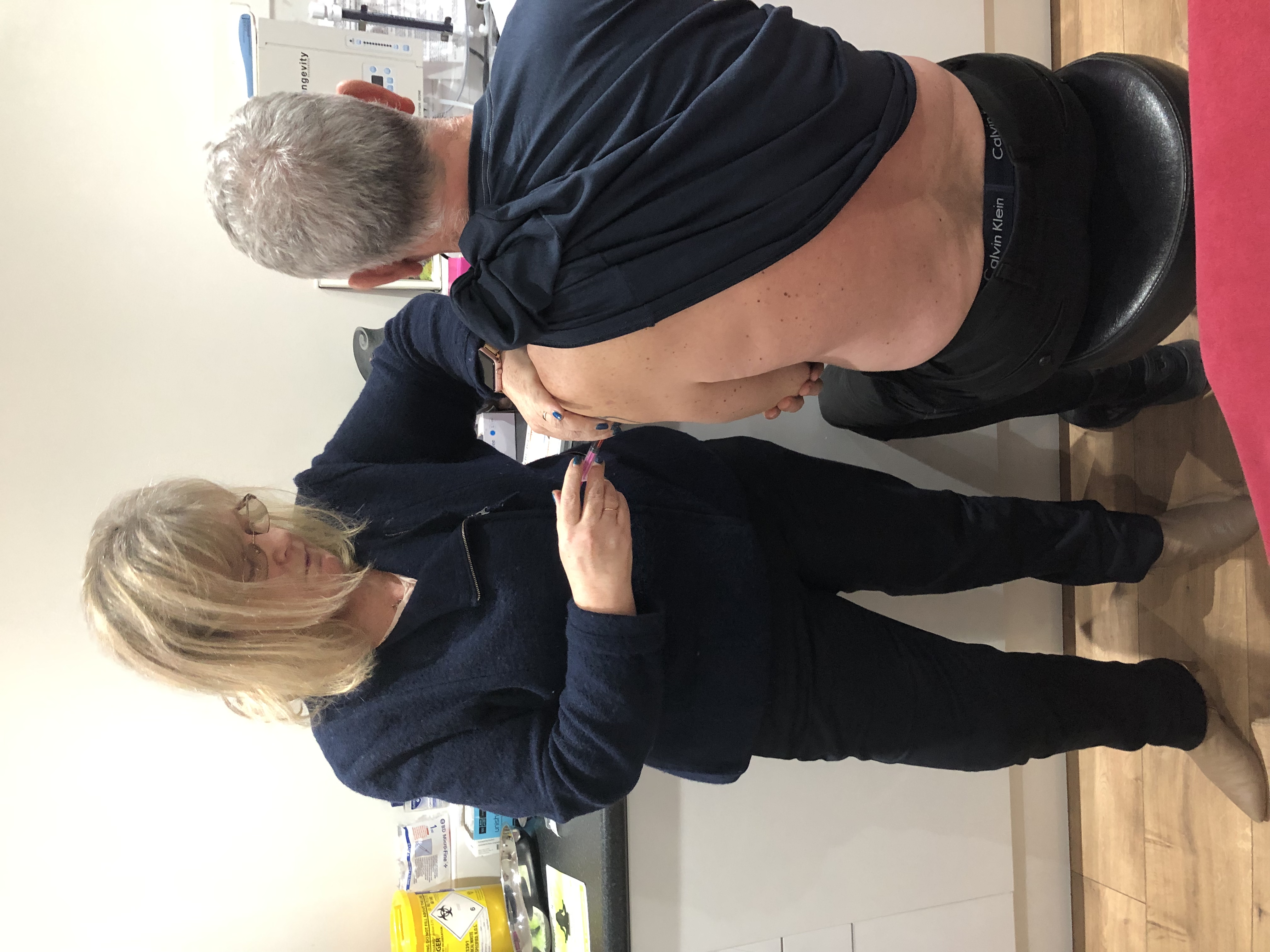
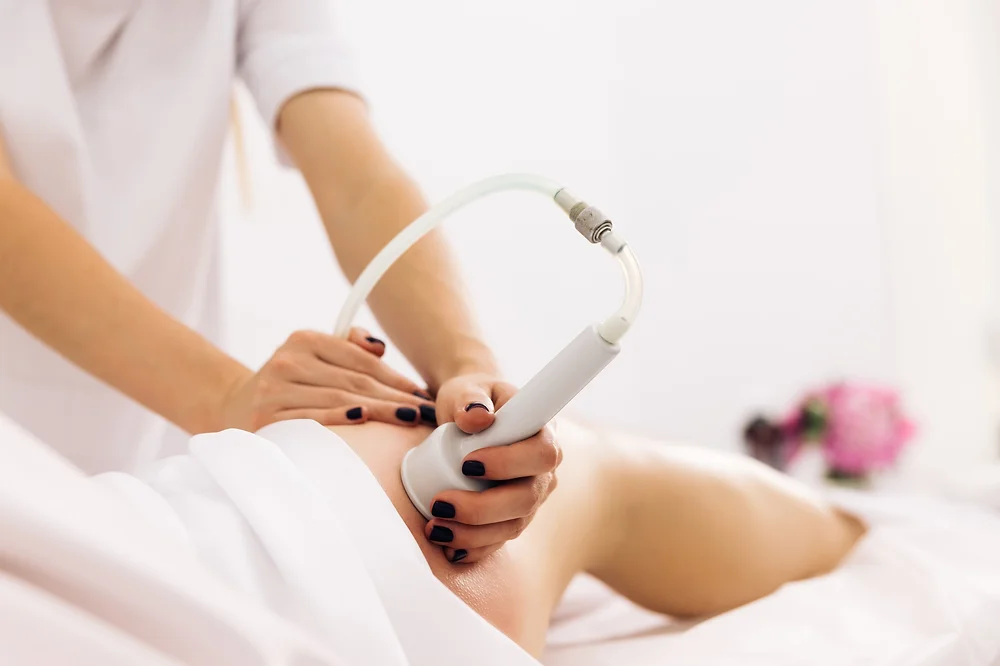
Cavitation and RF Course in person
By KBH Training Academy
Ultrasonic Cavitation and RF Course *machine is not included. You will be training on our equipment. About Treatments like ultrasonic cavitation can make it even trickier if you’re not even sure what that means or what it does. Put simply, ultrasonic cavitation is a weight-loss treatment that uses low-frequency sound waves to burst fat cells, which may result in lost inches on the treated areas and (ultimately) a slimmer figure. It’s been growing in popularity over the last few years because while traditional liposuction requires an invasive procedure to remove fat, this procedure doesn’t. What is ultrasonic cavitation? Ultrasonic cavitation is a simple procedure that relies on sound waves to flush fat from the body instead of intensive surgery. Course Content What is Cavitation Anatomy and physiology Health & Safety Consultations and Aftercare How to provide ultrasonic cavitation on body RF Training on body How does the course work? The course is divided into 2 parts, the first part is theoretical which you have to complete before you come for your practical training, and the second one is a practical assignment. The practical assignment is done on the day which will be agreed upon course purchase. You will spend around 2-3 hours practising on a model in our venue in London E106RA. Will I require a model? Yes, usually 1 model is required Do I Need Experience Before Booking a Course? We’re pleased to offer courses to people with lots of different experiences. However, previous experience nor qualifications are not necessary if you would like to enrol on our Course. Certificate You will receive an end of course certificate which is accredited by the cpd group and allows you to work on public Payment By paying for the course you agree to our terms and conditions.
GMP04: Good Manufacturing Practice for the Warehouse
By Zenosis
The warehouse plays a crucial role in a medicinal products factory. This module explains the requirements of Good Manufacturing Practice (GMP) for the warehouse, and how to comply with them.

SUB01: Orphan Drug Designation in the USA and Europe
By Zenosis
Medicines for the prevention, diagnosis, or treatment of rare diseases have become known as ‘orphan drugs’ because of their commercial unattractiveness. Development of such products is successfully encouraged through incentives offered by regulatory authorities. To qualify for important incentives, the sponsor of a drug must gain ‘orphan designation’ for its use in an indication. This module describes the requirements for orphan designation and how to apply for it in the USA and the European Economic Area.
GMP01: An Introduction to Good Manufacturing Practice for Medicinal Products
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. The rules may be written into law or set out in guidance documents from regulatory authorities. Regulators will not allow medicinal products to be placed, or to remain, on the market in their country unless the products can be shown to be manufactured in compliance with GMP. To this end, they carry out inspections of manufacturing plants. Companies that persistently commit serious breaches of GMP requirements have suffered huge fines.
PKPD02: Conducting Pharmacokinetic and Pharmacodynamic Studies
By Zenosis
This module extends the learner’s understanding of pharmacokinetic and pharmacodynamic studies from the basics described in our companion module PKPD01, An Introduction to Pharmacokinetics and Pharmacodynamics in Drug Development and Registration. It provides detail on a variety of aspects of such studies: design, sampling, data analysis, research in special populations, and bioequivalence testing.

ICT01: Compliance with Regulation 21 CFR Part 11 on Electronic Records and Electronic Signatures
By Zenosis
21CFR11 applies to records that are required to be submitted to the FDA, or that are subject to FDA inspection, and that are in electronic form – that is, as computer files. It applies to all computer systems used to create, modify, maintain, archive, retrieve, or transmit such records – from a humble spreadsheet program to a complex information management system.

CT06: Clinical Trial Monitoring: Site Evaluation and Setup
By Zenosis
The sponsor of a clinical trial needs to reach agreement with clinical investigators to conduct the trial. The suitability of investigators and their institutional sites, typically hospitals, has to be evaluated, and the trial has to be set up at each site. This module describes the processes involved, focusing particularly on the role of a Clinical Research Associate (CRA) employed or contracted by the sponsor to monitor the trial.
PV04: Signal Detection and Management in Pharmacovigilance
By Zenosis
This module provides a guide to signal detection and management for approved products. The subject is presented as a process comprising four stages: signal detection, signal validation, signal analysis and prioritisation, and risk assessment and minimisation.

Search By Location
- Medicine and Nursing Courses in London
- Medicine and Nursing Courses in Birmingham
- Medicine and Nursing Courses in Glasgow
- Medicine and Nursing Courses in Liverpool
- Medicine and Nursing Courses in Bristol
- Medicine and Nursing Courses in Manchester
- Medicine and Nursing Courses in Sheffield
- Medicine and Nursing Courses in Leeds
- Medicine and Nursing Courses in Edinburgh
- Medicine and Nursing Courses in Leicester
- Medicine and Nursing Courses in Coventry
- Medicine and Nursing Courses in Bradford
- Medicine and Nursing Courses in Cardiff
- Medicine and Nursing Courses in Belfast
- Medicine and Nursing Courses in Nottingham