- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
8158 Courses
ESS01: Essentials of EU and US Regulatory Affairs for Human Medicinal Products
By Zenosis
This foundation-level module is the ideal introduction for new entrants to the field of pharmaceutical regulatory affairs and compliance. It describes the principal requirements that must be satisfied to gain and maintain approval to market medicinal products in the USA and Europe. The legal framework and the roles of major players in regulation are presented. The life-cycle of a drug is outlined. The various procedures available for assessment and approval of products are described and their requirements outlined. Obligations to be fulfilled after marketing approval are discussed.

VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

SUB14: The Regulatory Pathway to Licensure of Follow-on Biologics (Biosimilars) in the USA
By Zenosis
The regulation of biological medicinal products is governed by different laws from those that apply to small-molecule synthetic drugs. Producing faithful copies of therapeutic proteins is more challenging than producing generic drugs. The US legal framework for the licensure of follow-on biologics, and accompanying regulatory guidance from the Food and Drug Administration (FDA), have been established only in recent years.

VAL04: Operational and Performance Qualification
By Zenosis
Having undergone Installation Qualification, before equipment can be used routinely in production, it needs to undergo Operational Qualification (OQ) and Performance Qualification (PQ). This module describes OQ and PQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
SUB03: The Mutual Recognition Procedure (MRP)
By Zenosis
his module describes the roles of the various players in the procedure, the sequence and duration of the stages involved, and the requirements on content, format and timing of submissions. It discusses the special issues that apply to generic products in the MRP.
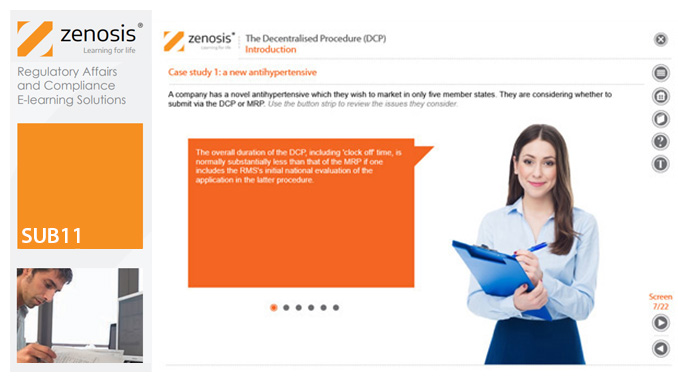
SUB11: The Decentralised Procedure (DCP)
By Zenosis
This module describes the roles of the various players in the procedure, the sequence and duration of the stages involved, and the requirements on content, format and timing of submissions. It discusses the special issues that apply to generic products in the DCP.
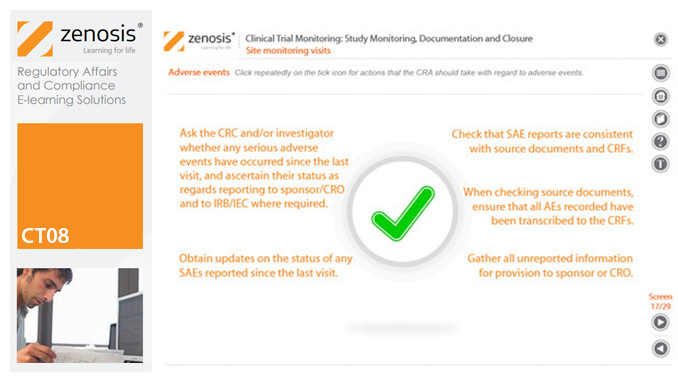
CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
By Zenosis
The sponsor of a clinical trial must arrange for it to be monitored throughout its duration to ensure that the rights and wellbeing of subjects are protected, the trial data are accurate, complete and verified from source documents, and the conduct of the trial complies with the study protocol, Good Clinical Practice and regulatory requirements. In this module we describe how a Clinical Research Associate (CRA) monitors an ongoing trial to its conclusion.
Description Basic Electricity Diploma Introducing the Basic Electricity Diploma, an expertly designed online course crafted to impart foundational knowledge and skills surrounding the realm of electricity. This course is tailored to meet the ever-evolving needs of individuals seeking to understand the core principles of electricity, from its safe application to advanced troubleshooting techniques. Every budding electrician, enthusiast, or professional aiming to refresh their knowledge should consider the Basic Electricity Diploma as an essential stepping stone. The content is not just a compilation of theory; it aims to prepare the learner for real-world application. The course starts with a pivotal module on Electrical Safety. Ensuring safety is not just a protocol, but a crucial part of working with electricity. This module ensures that students understand the fundamental safety protocols and potential hazards to be mindful of when working with electrical systems. Understanding the pathways electricity takes is vital. Circuit Analysis will provide learners with knowledge about how circuits function, the different components within them, and the importance of each in a fully functional system. Ever wondered about the motors that power so many of our daily appliances? The section on Electric Motors demystifies this by breaking down their functioning, types, and applications. Simultaneously, the Electrical Power Distribution Systems module introduces learners to the intricate systems in place to deliver electricity from its source to our homes and businesses. The distinction between AC and DC Circuits is foundational to anyone in the electrical field. This course ensures a thorough grasp of both, detailing their individual characteristics, benefits, and applications. Transformers and Capacitors play pivotal roles in the world of electricity. This section of the Basic Electricity Diploma discusses their functionality, importance, and the fundamental physics behind them. With the fast-paced technological advancements, Electrical Control Systems have become more sophisticated and intricate. This module covers the basics of these systems, offering insight into their operation and real-world application. No course on electricity would be complete without discussing the various Electrical Machines and Devices. This module provides an introduction to the most common machines and devices, from generators to common household appliances, shedding light on their workings. Adhering to the right guidelines is paramount. The Electrical Code and Standards module enlightens learners about the critical standards, both national and international, ensuring that all electrical work aligns with the highest levels of safety and quality. Lastly, the Troubleshooting Electrical Systems unit empowers learners with the skills and knowledge to diagnose and rectify common electrical issues, making them an invaluable asset in any electrical role. To sum it up, the Basic Electricity Diploma is a comprehensive online course designed to provide a robust foundation in electricity. From fundamental safety protocols to advanced topics like troubleshooting, this course covers it all, making it a must-have for anyone eager to embark on or advance their journey in the electrical domain. Equip yourself with the knowledge you need and take your electrical understanding to the next level. Register today! What you will learn 1:Electrical Safety 2:Circuit Analysis 3:Electric Motors 4:Electrical Power Distribution Systems 5:AC and DC Circuits 6:Transformers and Capacitors 7:Electrical Control Systems 8:Electrical Machines and Devices 9:Electrical Code and Standards 10:Troubleshooting Electrical Systems Course Outcomes After completing the course, you will receive a diploma certificate and an academic transcript from Elearn college. Assessment Each unit concludes with a multiple-choice examination. This exercise will help you recall the major aspects covered in the unit and help you ensure that you have not missed anything important in the unit. The results are readily available, which will help you see your mistakes and look at the topic once again. If the result is satisfactory, it is a green light for you to proceed to the next chapter. Accreditation Elearn College is a registered Ed-tech company under the UK Register of Learning( Ref No:10062668). After completing a course, you will be able to download the certificate and the transcript of the course from the website. For the learners who require a hard copy of the certificate and transcript, we will post it for them for an additional charge.

Search By Location
- Medicine and Nursing Courses in London
- Medicine and Nursing Courses in Birmingham
- Medicine and Nursing Courses in Glasgow
- Medicine and Nursing Courses in Liverpool
- Medicine and Nursing Courses in Bristol
- Medicine and Nursing Courses in Manchester
- Medicine and Nursing Courses in Sheffield
- Medicine and Nursing Courses in Leeds
- Medicine and Nursing Courses in Edinburgh
- Medicine and Nursing Courses in Leicester
- Medicine and Nursing Courses in Coventry
- Medicine and Nursing Courses in Bradford
- Medicine and Nursing Courses in Cardiff
- Medicine and Nursing Courses in Belfast
- Medicine and Nursing Courses in Nottingham