- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
28780 Courses
Customer Service Masterclass - An Unforgettable Support Experience
By Compete High
ð Elevate Your Customer Service to Extraordinary Heights! ð Are you ready to revolutionize the way you interact with your customers? Welcome to the 'Customer Service Masterclass - An Unforgettable Support Experience' - the ultimate online course designed to transform your customer service from ordinary to extraordinary! ð Why Choose Our Masterclass? ⨠Create Unforgettable Experiences: Learn the art of crafting memorable interactions that leave a lasting impression on your customers. From first contact to issue resolution, discover the secrets to building meaningful connections. â Master Proactive Problem-Solving: Anticipate your customers' needs before they even realize it! Uncover proven strategies to address concerns efficiently and effectively, turning potential problems into opportunities to showcase your stellar service. ð Navigate the Digital Landscape with Ease: In today's digital age, customer service goes beyond face-to-face interactions. Gain insights into managing online inquiries, social media interactions, and virtual support channels with finesse. ð¥ Boost Customer Satisfaction and Loyalty: Happy customers are loyal customers. Uncover the techniques that will not only meet but exceed customer expectations, fostering loyalty that lasts a lifetime. ð Measure and Improve: Dive into the world of analytics and feedback to continuously enhance your service. Discover how to turn data into actionable insights, driving ongoing improvements for an ever-evolving customer experience. ð What You'll Learn: The Psychology of Customer Satisfaction Effective Communication Strategies Handling Challenging Situations with Grace Implementing Proactive Customer Service Harnessing Technology for Seamless Support Building a Customer-Centric Culture within Your Team ð Exclusive Bonuses: Enroll now and receive: ð Comprehensive Course Materials ð£ï¸ Access to a Thriving Community of Like-Minded Professionals ð Practical Tools and Templates for Immediate Implementation ð©âð» Who Should Enroll: Customer Service Representatives Support Team Leaders Entrepreneurs and Small Business Owners Anyone Passionate about Delivering Exceptional Service ð¡ Ready to Stand Out in a Crowded Marketplace? Don't miss out on the chance to take your customer service to new heights. Join the 'Customer Service Masterclass' and unlock the key to creating an unforgettable support experience for your customers. Enroll now and let excellence become your standard! ð Transform today, thrive tomorrow! ð Course Curriculum

September 2025 Fundamentals Organisation & Relationship Systems Coaching Training
By CRR UK
CRRUK equips professionals with the concepts, skills and tools to build conscious, intentional relationships, and to coach relationship systems of any size.

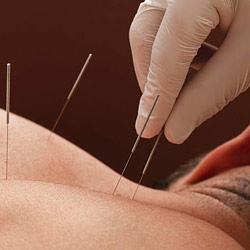
Course Summary Enhance your manual therapy practice with Dry Needling. This comprehensive course, led by experienced Osteopath Becky Tyler, provides the essential knowledge and practical skills to confidently integrate Dry Needling into your treatments. Course Highlights: Expert Instruction: Learn from Becky Tyler, an experienced Osteopath, ensuring high-quality, clinically relevant training. Comprehensive Curriculum: Covering foundational theory, practical demonstrations, and extensive hands-on practice, you’ll master trigger point Dry Needling techniques. Practical Focus: Gain proficiency through ample practical sessions, allowing you to refine your skills and apply techniques effectively. Clinical Application: Learn to strategically apply Dry Needling for muscular pain relief, understanding both when and when not to use it. Professional Development: This CPD course aims to develop competent and confident practitioners, enabling you to offer a more comprehensive treatment approach. Course Structure: Online Pre-Learning: Foundational theoretical knowledge. Two-Day In-House Practical Training: Advanced theory and intensive practical application. Post-Course Case Study: Demonstration of clinical reasoning and application. Course Content Introduction History of Acupuncture About Needles Acupuncture Application Effects of Acupuncture Contractions & Contraindications Practical Application Conditions Covered include; Plantar-Fasciitis, Rotator Cuff, Tennis and Golders Elbow, Lower Back Pain/Pathologies, Local Muscular Pain, Muscles Trigger points and much more! Course Prerequisites Physiotherapists Osteopaths Chiropractors Manual Therapists Sports Therapists Sports Massage Therapists (Must be Level 4 qualified or above) We may ask you to provide a copy of your qualification. Assessment Your competency will be assessed through: Online Pre-Learning Quizzes: To ensure comprehension of foundational knowledge. Practical Skills Observations: Continuous assessment of practical technique during training. Post-Course Case Study: A comprehensive evaluation of your ability to apply Dry Needling principles in a clinical context. Certification On completion of this course you will receive a Certificate of Competency in Dry Needling Once completed you will be able to add Dry Needling therapy on to your own indemnity insurance, You will also need to apply for a license from your local council Venue BTST Academy & Clinic, Holly Farm, Clipstone Road, Edwinstowe, Nottingham, NG21 9JD Course Times Start 9.30am – Finish 4:30pm Course Price £ 325 Tutor Becky Tyler BOst, PGCertSPOP, DipSMT Course Terms & Conditions: Click here for the terms and conditions. Course Accreditation Accredited by Active IQ What our Learners say: Gemma Parker; After completing a 2 day Dry Needling course with Becky at her academy, I can highly recommend her amazing facilities and brilliant training. Becky is a great teacher and everyone involved with the academy are super friendly, the location is just stunning too. I thoroughly enjoyed the whole experience and will be booking another course real soon. Dan Green; Excellent facility, great course and very knowledgable instructor. Very highly recommended All reviews taken from our Google Reviews
Practical Sales Skills 1 Day Workshop in Paisley
By Mangates
Practical Sales Skills 1 Day Workshop in Paisley

Practical Sales Skills 1 Day Workshop in High Wycombe
By Mangates
Practical Sales Skills 1 Day Workshop in High Wycombe

Search By Location
- experience; Courses in London
- experience; Courses in Birmingham
- experience; Courses in Glasgow
- experience; Courses in Liverpool
- experience; Courses in Bristol
- experience; Courses in Manchester
- experience; Courses in Sheffield
- experience; Courses in Leeds
- experience; Courses in Edinburgh
- experience; Courses in Leicester
- experience; Courses in Coventry
- experience; Courses in Bradford
- experience; Courses in Cardiff
- experience; Courses in Belfast
- experience; Courses in Nottingham
