- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
8158 Courses
Lymphatic Drainage Massage Training Course
By Cosmetic College
Join our one-day professional training course in Manual Lymphatic Drainage (MLD), a unique massage technique known for its detoxifying, calming, and pain-relieving benefits, in addition to boosting the immune system. Despite its profound effects, it's straightforward to master with guidance from our skilled instructors. The massage technique is particularly useful for the treatment of lymphoedema and swelling that is often seen in response to cancer treatments such as radiotherapy. It's specialised pumping technique can be used to prevent or treat lymphoedema and improve lymph drainage. Join our professional one-day Manual Lymphatic Drainage (MLD) Practitioner course to master a massage technique renowned for its therapeutic benefits, including detoxification, relaxation, pain relief, and immune support. With expert tutors, learn this effective, gentle method to address a range of conditions, from lymphoedema to stress. Our focused, small-class setting ensures personalised instruction, making our training center a top choice in the UK for developing advanced skills in MLD. Why Choose Our Training? Immediate Impact:Learn treatments that offer visible results from the first session. High Demand Skills: Master a technique with growing client demand, ensuring your services are always in demand. Expert Instructors: Benefit from hands-on training by industry leaders with in-depth experience in aesthetic medicine. Course prerequisites This course is suitable for: No previous experience is necessary NVQ Level 3 in beauty therapy, ITEC or HND is desirable Qualifications in sports massage or full body massage is desirable Course structure You are required to complete 20 hours of theory study via our accessible e-learning portal and 4 practical hours onsite. All courses are kept intimate with a maximum of 6 learners to a class. Areas covered within the course: Explore the anatomy and roles of the lymphatic system, focusing on its components such as lymphocytes, tissues, vessels, nodes, ducts, capillaries, and the spleen, to understand its crucial role in immunity and fluid balance. Key lymph nodes located throughout the body. Overview of the blood vessels of head and neck Relationship between blood and lymph History of Manual Lymphatic Drainage Massage Contraindication to Manual Lymphatic Draining Massage treatment Guidance on manual lymphatic drainage massage techniques, detailing the four foundational strokes and other methods. These techniques are designed to be versatile, allowing for application across any body part and integration into existing massage practices

Radio Frequency Skin Tightening Training Course
By Cosmetic College
When attending our Radio frequency course, our students are taught the core knowledge behind the technology enabling them to offer safe fat reduction and skin tightening for the face and body. Course prerequisites Minimum 18 years of age Good command of English Be able to learn independently A strong desire to build a career in aesthetics Previous skin and facial training are desirable we suggest that learners new to the industry enrol on our facial and skincare course prior to enrolling on our ClinicCare skin peel course. Course agenda A mixture of online study, virtual lectures and onsite practical sessions. Areas covered in this course: Industry regulation Insurance Client care/consultation Contra-indications and precautions Radio frequency skin tightening core knowledge Aftercare Maintenance Further treatment advice Promotion of this exciting treatment Practical demonstration Practice sessions Practical on-going assessments Health and Safety
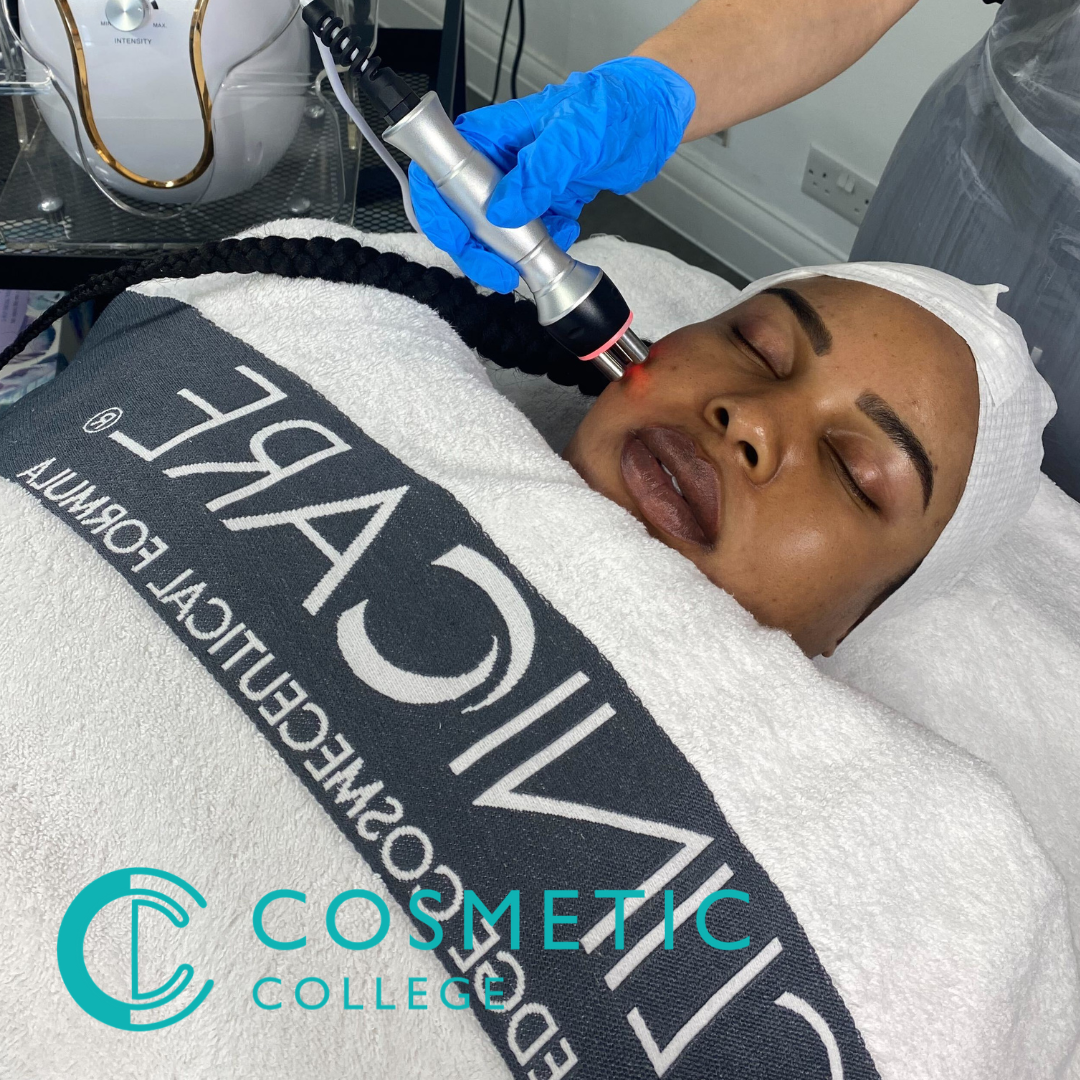
Aqualyx & Deso Fat Dissolving Training
By Cosmetic College
Aqualyx is one of the leading brands that is used for fat dissolving. Aqualyx can be used on the face and body to great effect. Just one session can dramatically reduce fat deposits and return a more youthful and defined facial contour for small areas such as the jowls and chin. For larger areas like tummies, bingo wings and bums, clients may need up to 5 sessions to see dramatic results. Aqualyx & Deso are water-based solutions injected into the fatty tissue, surround the cells and destroy them. The remains of the fat cells are then excreted by the body safely as waste. Aqualyx is used for people who want to get rid of stubborn areas of fat in their faces and bodies and is clinically proven for these purposes. Course Entry Requirements: This course is suitable for both medics and non-medics. You can enrol on this training course if you meet one of the following: NVQ Level 3 in beauty therapy, ITEC or HND Medically qualified as a nurse, doctor or dentist with current registration with the NMC, GMC or GDC. 12 Months of Needling Experience or 6 Months of Needling Experience and Anatomy & Physiology Level 3 or Above. Course Structure: 20 hours of pre-study E-learning and 1 practical training Course Agenda: Areas covered within the course: Anatomy and physiology of the skin and tissues Infection control Sharps and hazardous waste training First aid and anaphylaxis training Pre-study fat-dissolving theory training Practical training Clinical set up Professional live demonstrations Course Benefits Benefits for Students Specialised Training: Our course provides specialised training in Aqualyx and Deso fat dissolving treatments. You will learn the techniques, protocols, and safety considerations involved in these procedures. This specialised training will enhance your skill set and allow you to offer advanced fat dissolving treatments to your clients. Practical Experience: We prioritise hands-on learning to ensure that you gain practical experience in administering Aqualyx and Deso fat dissolving treatments. Under the guidance of experienced instructors, you will have the opportunity to practice these procedures on models. This practical training will increase your confidence and competence in performing the treatments effectively. Expanded Service Offering: By enrolling in our training course, you will expand your service offering as an aesthetics practitioner. Aqualyx and Deso fat dissolving treatments are highly sought after by clients who want to target localised fat deposits non-surgically. Adding these treatments to your repertoire will attract new clients and provide existing clients with more comprehensive solutions. Professional Advancement: Completing our Aqualyx & Deso Fat Dissolving Training Course will contribute to your professional advancement. You will gain in-depth knowledge about fat dissolving treatments, build expertise in these procedures, and enhance your professional credibility. This can lead to career growth opportunities, such as working in reputable clinics, establishing your own practice, or even training other professionals in the field. Benefits for Clients Targeted Fat Reduction: Clients seeking Aqualyx and Deso fat dissolving treatments desire targeted fat reduction in specific areas of their body. By enrolling in our training course, you will acquire the skills to accurately assess and administer these treatments, effectively helping clients reduce unwanted fat deposits in a non-surgical manner. Non-Invasive Solution: Aqualyx and Deso fat dissolving treatments provide a non-invasive alternative to surgical procedures for fat reduction. Clients benefit from a less invasive approach that involves fewer risks, minimal downtime, and reduced recovery periods. Offering these non-invasive treatments can attract clients who prefer a non-surgical solution to their aesthetic concerns. Customised Treatment Plans: As a trained practitioner, you will be able to create customised treatment plans for your clients. By assessing their unique needs and goals, you can develop tailored protocols and treatment strategies. This personalised approach ensures that clients receive individualised care and optimal results. Earning Potential The demand for Aqualyx and Deso fat dissolving treatments continues to grow. By enrolling in our training course and becoming proficient in these procedures, you can significantly increase your earning potential. Clients are willing to invest in effective fat reduction treatments, and as a skilled practitioner, you can attract a larger clientele and set competitive pricing for your services. Satisfied clients often become repeat customers and refer others, further boosting your earning potential. Enrolling in our Aqualyx & Deso Fat Dissolving Training Course not only benefits you as a student but also provides valuable advantages for your clients and potential earning potential in the aesthetics industry. Take the opportunity to expand your expertise and offer innovative solutions to clients seeking fat reduction treatments. Frequently Asked Questions Is this course suitable for beginners or those with prior experience? Aqualyx & Deso Fat Dissolving Training Course is designed for aesthetics practitioners who already have a foundation in basic injection techniques and experience in the field. Prior experience in administering injections is essential to enrol in this advanced course. Will I receive a certification upon completion? Yes, upon successfully completing our training course, you will receive a certification in Aqualyx & Deso Fat Dissolving. This certification acknowledges your advanced skills and expertise in administering these fat dissolving treatments. It can enhance your professional reputation and provide a competitive edge in the aesthetics industry. What topics are covered in the course curriculum? Our course curriculum covers various topics related to Aqualyx and Deso fat dissolving treatments. These include product knowledge, patient selection and assessment, injection techniques, treatment protocols, managing complications, and post-treatment care. The curriculum is designed to provide you with a comprehensive understanding of the Are there any hands-on training opportunities? Yes, our Aqualyx & Deso Fat Dissolving Training Course includes hands-on training sessions. Under the supervision of experienced instructors, you will have the opportunity to practice and refine your skills in administering these fat dissolving treatments. This practical experience is vital for your professional development. Will I have access to ongoing support after completing the course? Absolutely! We provide ongoing support to our students even after they complete the training course. Our instructors and support staff are available to answer any questions, provide guidance, and offer assistance as you navigate your career in Aqualyx and Deso fat dissolving treatments. We are committed to supporting your continued growth and success. Are there financing options available for the course? We offer flexible payment options and financing plans to make our Aqualyx & Deso Fat Dissolving Training Course more accessible. Please reach out to our admissions team for detailed information on available payment options and financing plans.
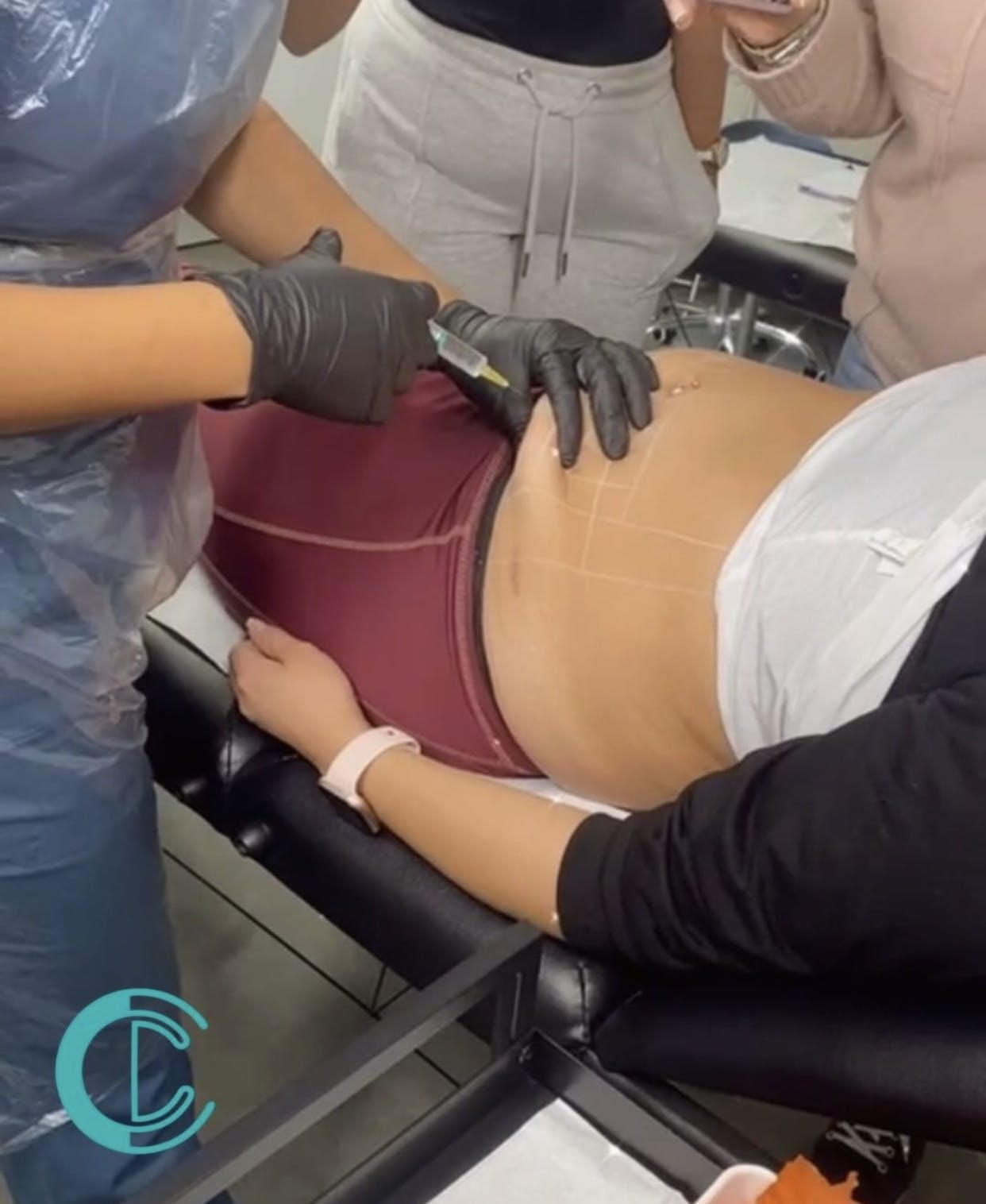
Lidocaine Infusion Training Course
By Cosmetic College
Course Prerequisites This course is suitable for those that have completed a Foundation Dermal Fillers course. Course Pre Study 8 hours of online study An online assessments Half a day of Practical training Course Structure This course is held for 3 hours of intensive theory and practical sessions. All courses are kept intimate with a maximum of 4 students per course. The Lidocaine Infusion course consists of the following theoretical and practical components. Theory: Structure of Lidocaine Suitability for Lidocaine Nerves Side effects Consent forms Aftercare Managing expectations Client consultation Hygiene, sharps disposal and legal aspects Practical Components: Injection techniques Live demonstration One live model
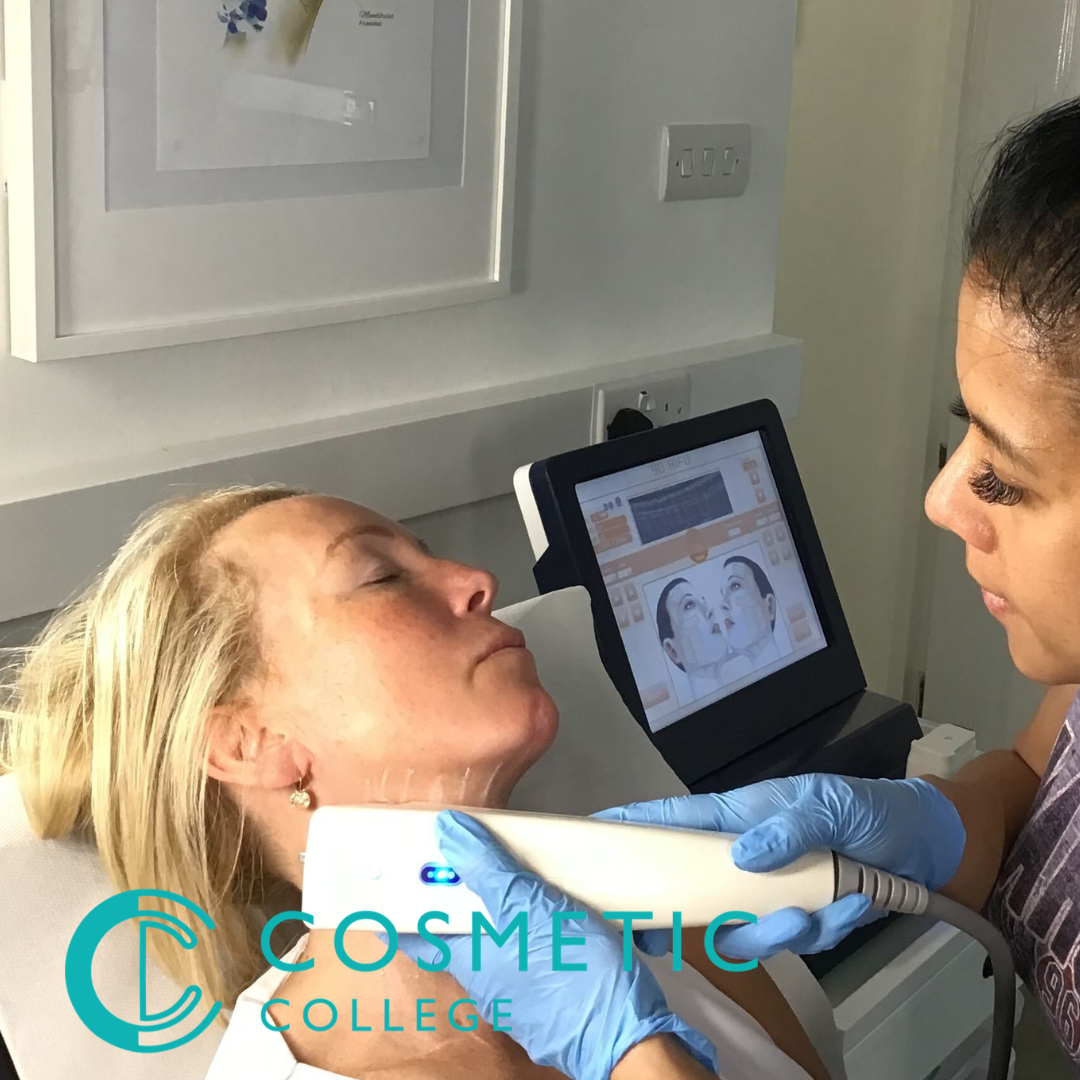
High Intensity Focused Ultrasound (HIFU) for Face and Neck
By Cosmetic College
Age and gravity effects on our skin are offset by non-invasive HIFU treatment using the body's own recuperative powers to lift the skin of the face, neck, under the chin, and décolleté, lift eyebrows and smooth wrinkles and lines. It is safe and effective. HIFU treats tissue otherwise only reached by surgery. HIFU can go beneath the surface of the skin without breaking the outer skin and reach into the dermis and SMAS layer. It is suitable for men as well as women. This training course for HIFU Non-Surgical Facial Therapy is specially developed for the more experienced facial specialist who wants to offer HIFU Non-Surgical Facial Therapy as a new treatment on their menu. Course prerequisites This course is suitable for both medics and non-medics. Level 3 in Beauty Therapy or equivalent is desirable Good command of English A minimum of 18 years Previous skin and facial training are desirable we suggest that learners new to the industry enrol on our facial and skincare course prior to enrolling on our ClinicCare skin peel course. Course structure A mixture of online study, virtual lectures and an onsite practical session. All courses are intimate with four learners in class 2-1 ratio. Areas covered within the course: Health and safety Anatomy and Physiology Fat types Fat Distribution Role of fat in the body The science behind HIFU for Fat Reduction The science behind HIFU for skin laxity Stages of skin laxity Collage Elastin Fibroblasts Wound healing process Selecting different equipment and the benefits associated Cartridge type Areas suitable for treatment Treatment duration Protocols including frequency, intensity etc Benefits for the client and clinic Contra indications and actions Client suitability and assessment for treatment Risks and side effects Training is complimentary when you purchase the Beautier 4D HIFU machine. Contact us for the course and machine package prices.
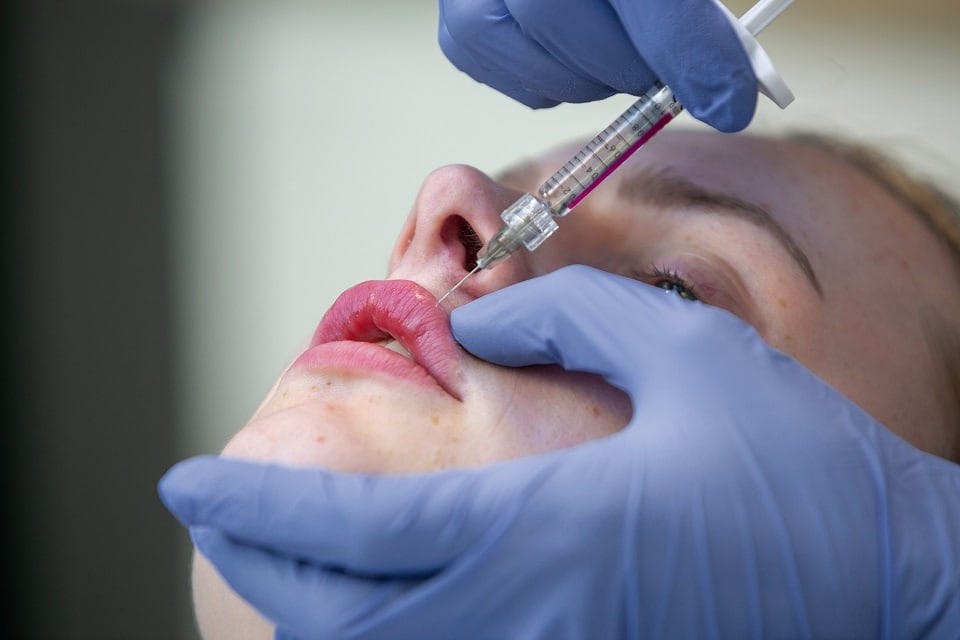
Ultrasound Cavitation Training Course
By Cosmetic College
Cavitation, also known as Ultrasound Cavitation, is a treatment that has been around for a number of years now, as a non-surgical alternative to liposuction for the reduction of Fat. Cavitation uses ultrasound waves to break down the excess fat. We call it the 'non-surgical liposuction' because there is no need for anesthesia, no pain, no incision and no recovery time. The ultrasound waves are delivered through the surface of the skin via an ultrasound probe. Cavitation can be dangerous if not used correctly due to the depth of penetration of the ultrasound beam but with the correct training and understanding safe practices can be used to ensure great results to remove excess fat without the need for surgery. Cavitation can also be used in the treatment of cellulite, skin tightening and stretch marks. Cavitation is a great treatment on its own but can also be combined with other treatments to increase its effectiveness such as Radio-frequency for skin tightening and Shock-wave therapy for cellulite. Course prerequisites Minimum 18 years of age Good command of English Be able to learn independently Course structure You are required to complete 20 hours of theory study via our accessible e-learning portal and 5 practical hours onsite. All courses are kept intimate with a maximum of 6 learners in a class. Areas covered within the course: Core knowledge Health and safety and data protection Confidentiality, privacy Hygiene and sterilisation Equipment maintenance and set-up Cellulite and full understanding Body types and fat tissue Fat assessment including BMI Candidates for cavitation Cellulite History of ultrasound and uses Principle of cavitation Objections and how it works Benefits of cavitation Consultation, contra-indications, skin sensitivity, treatment times, safety, risk, side effects, recovery and aftercare Live demo

Consent Training and Community Health Agent - Double Endorsed Certificate
By Imperial Academy
2 QLS Endorsed Course | CPD Certified | Free PDF + Hardcopy Certificates | 80 CPD Points | Lifetime Access

Community Health Agents and Social Care Management - Double Endorsed Certificate
By Imperial Academy
2 QLS Endorsed Course | CPD Certified | Free PDF + Hardcopy Certificates | 80 CPD Points | Lifetime Access

2-Day Transition from CFR to FREC 3
By NR Medical Training
Qualsafe Awards has recognised the invaluable experience and training that CFRs bring to the table. As a result, they've introduced a bespoke 2-day FREC3 RPL Course, specifically designed for learners like you who already hold one of the approved qualifications. This course recognises your prior learning, allowing you to fast-track your way to the FREC 3 qualification.

Tired of browsing and searching for a Nursing Assistant (Nurse Prescribing, Phlebotomy, Medication) course you are looking for? Can't find the complete package that fulfils all your needs? Then don't worry as you have just found the solution. Take a minute and look through this extensive bundle that has everything you need to succeed. After surveying thousands of learners just like you and considering their valuable feedback, this all-in-one Nursing Assistant (Nurse Prescribing, Phlebotomy, Medication) bundle has been designed by industry experts. We prioritised what learners were looking for in a complete package and developed this in-demand Nursing Assistant (Nurse Prescribing, Phlebotomy, Medication) course that will enhance your skills and prepare you for the competitive job market. Also, our experts are available for answering your queries on Nursing Assistant (Nurse Prescribing, Phlebotomy, Medication) and help you along your learning journey. Advanced audio-visual learning modules of these Nursing Assistant (Nurse Prescribing, Phlebotomy, Medication) courses are broken down into little chunks so that you can learn at your own pace without being overwhelmed by too much material at once. Furthermore, to help you showcase your expertise in Nursing Assistant (Nurse Prescribing, Phlebotomy, Medication), we have prepared a special gift of 1 hardcopy certificate and 1 PDF certificate for the title course completely free of cost. These certificates will enhance your credibility and encourage possible employers to pick you over the rest. This Nursing Assistant (Nurse Prescribing, Phlebotomy, Medication) Bundle Consists of the following Premium courses: Course 01: Level 3 Diploma in Nursing Assistant Complete Training Course 02: Public Health Course 03: Medical Law Course 04: Nurse Prescribing Diploma Course 05: Phlebotomy Course 06: Clinical Training for Nurses and Carers - Catheterisation Course 07: Medication Administration Level 4 Course 08: GDPR in Healthcare Course 09: Cardiac (Heart) Care Course 10: Diabetes Care Diploma Course 11: Wound Care Level 2 Course 12: Understanding Autism Awareness and Diagnosis Level 2 Course 13: Nutrition and Diet Awareness Course 14: First Aid Training Enrol now in Nursing Assistant (Nurse Prescribing, Phlebotomy, Medication) to advance your career, and use the premium study materials from Apex Learning. How will I get my Certificate? After successfully completing the Nursing Assistant course, you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (For The Title Course) Hard Copy Certificate: Free (For The Title Course) The bundle incorporates basic to advanced level skills to shed some light on your way and boost your career. Hence, you can strengthen your Nursing Assistant (Nurse Prescribing, Phlebotomy, Medication) expertise and essential knowledge, which will assist you in reaching your goal. Curriculum of Nursing Assistant Bundle Course 01: Level 3 Diploma in Nursing Assistant Complete Training Module 1: Introduction to PA - Personal Assistant Diploma Module 2: Working in Different Healthcare Settings Module 3: Legal, Standards and Professional Aspects of Ethical Practice: Part - 1 Module 4: Legal, Standards and Professional Aspects of Ethical Practice: Part - 2 Module 5: Effective Communication in Nursing Module 6: Health and Safety in Nursing Module 7: Hygiene in Nursing Module 8: Infection Control Module 9: Asepsis in Nursing Module 10: Medication Administration in Nursing Module 11: Understanding the Immune System in Nursing Module 12: Rest and Sleep Management in Nursing Module 13: Mobility and Immobility Issues of Patients in Nursing Module 14: Pain Management for Nurses Module 15: Nutrition in Nursing Module 16: Fluid and Electrolyte Balance Module 17: Assisting with Elimination Module 18: Oxygenation in Nursing Course 02: Public Health Module 01: Introduction to Public Health Module 02: Principles of Public Health Module 03: Understanding Epidemiology Module 04: Disease Control Module 05: Understanding Measures of Disease Frequency Module 06: Maternity and Childbirth Module 07: Environment and Public Health Module 08: Health System and Policy Module 09: Public Health and Ethics Course 03: Medical Law Module 01- An Introduction to Medical Law Module 02- Legislation on Access to Health, Medical Report, Treatment Module 03- Legislation on Adult Support Module 04- Legislation on Public Health and Health Service (Part 1) Module 05- Legislation on Public Health and Health Service (Part 2) Module 06- Legislation on Public Health and Health Service (Part 3) Module 07- Legislation on Public Health and Health Service (Part 4) Module 08- Legislation on Coronavirus Module 09- Legislation on Mental Health (Part 1) Module 10- Legislation on Mental Health (Part 2) Module 11- Legislation on Abortion Module 12- Other Legislation (Part 1) Module 13- Other Legislation (Part 2) Course 04: Nurse Prescribing Diploma Module 1: Introduction to Nurse Prescribing Module 2: Legal and Ethical Aspects of Prescribin Module 3: Medicine Management Module 4: Anxiety Disorders in Adults Module 5: Liver Diseases: Diagnosis & Prescribing Module 6: Common Problems: Pharmacological Management Module 7: Anti-Microbial Prescribing Module 8: Cardiac Problems and Prescribing Module 9: Neurological Problems and Prescribing Module 10: Palliative Cares Module 11: Prescribing in Pregnancy and Lactation Module 12: Prescribing for Older People Module 13: Prescribing Children's Module 14: Common Medicines in Use Module 15: Challenges and Future of Nurse Prescribing Course 05: Phlebotomy Module 01: Introduction to Phlebotomy Module 02: Blood Circulation, Function, and Composition Module 03: Phlebotomy Equipment Module 04: Routine Venipuncture Module 05: Venipuncture Complications and Pre-Examination Variables Module 06: Dermal Puncture Module 07: Quality Assessment and Management in Phlebotomy Module 08: Special Blood Collection Procedure Module 09: Infection Control and Risk Management Course 06: Clinical Training for Nurses and Carers - Catheterisation Module 1: Introduction to Urinary Catheterisation Module 2: The Urinary System Module 3: Patients' Guide for Catheterisation Module 4: Guidance for Nursing and Care Staff Module 5: Protocol for Female Catheterisation Module 6: Protocol for Male Catheterisation Module 7: Protocol for Handling Catheter & Catheter Bags Course 07: Medication Administration Level 4 Module 01: Introduction to Safe Handling of Medicines Module 02: Legislation and guidance of medication management Module 03: Principles of Safe and Appropriate Handling of Medicines Module 04: Medication Risk Assessment Guidance Module 05: Handling Medicines in Social Care Settings Module 06: Consent to Treatment Module 07: Requirements for Specific Services Module 08: Levels of Care and Support Module 09: Procedure for Handling Medication Module 10: The Six Rights of Medication Administration Module 11: Covert Administration of Medication Module 12: Recording Procedures Module 13: Storage of Medication Module 14: Transfer and Disposal of Medication Module 15: Drug Formulation and Ways of Taking It Course 08: GDPR in Healthcare Module 01: Introduction to GDPR Module 02: GDPR and Healthcare Setting Module 03: General Data Protection Regulations Explained Module 04: Lawful Basis for Preparation Module 05: Responsibilities and Obligations Module 06: Electronic Medical Records Module 07: Rights and Breaches Course 09: Cardiac (Heart) Care Introduction & First Concepts What is A Heart Attack & Complications Emergency Care For A Heart Attack Risk Factors & Lifestyle Choices Heart Remedies Final Tips & Suggestions Course 10: Diabetes Care Diploma Module 01: What is Diabetes Module 02: The Diabetes Challenge Module 03: The Cost of Diabetes in Social Care Module 04: Type 1 Diabetes Module 05: Type 2 Diabetes Module 06: Type 2 Diabetes Treatments Module 07: Prediabetes Module 08: Gestational Diabetes Module 09: Other Types of Diabetes Module 10: Hypoglycaemia Module 11: Hyperglycaemia and Hyperosmolar Hyperglycaemic State Module 12: Glossary of Diabetes Terms Course 11: Wound Care Level 2 Module 01: The Wound Healing Process & Factors Delaying Wound Healing Module 02: Types of Wounds and Their Treatment Module 03: Dressing Module 04: Wound Assessment Module 05: Glossary Course 12: Understanding Autism Awareness and Diagnosis Level 2 Module 1: Autism Spectrum Disorder (ASD) Module 2: Learning Difficulties Related to Autism Module 3. Autism Diagnosis Module 4. Cognitive Approaches in Autism Module 5. Dealing with Autistic Individuals Module 6. Other Considerations for Dealing with Autistic Individuals Module 7. Engagement in Autism Awareness Course 13: Nutrition and Diet Awareness Nutrition and Diet Awareness Overview Nutritional Details What to Avoid & Problem Areas Beyond Food Putting It All Together Course 14: First Aid Training Introduction to Workplace First Aid Legal Framework for Workplace First Aid Incident Management at Work Primary Survey Secondary Survey Basic First-Aid Techniques Dealing with Minor Injuries at the Workplace Secondary Illness Loss of Responsiveness and CPR Secondary Illness Breathing Problems Secondary Illnesses and Injuries Dealing With Fractures and Dislocations Call for an Emergency CPD 140 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone from any background can enrol in this Nursing Assistant (Nurse Prescribing, Phlebotomy, Medication) bundle. Please note: This course doesn't entitle you to practice as a professional in this specific field. Rather, this course will assist you in understanding the fundamentals so that you can improve your knowledge in the relevant field. Requirements Our Nursing Assistant (Nurse Prescribing, Phlebotomy, Medication) course is fully compatible with PCs, Macs, laptops, tablets and Smartphone devices. Career path Having this Nursing Assistant (Nurse Prescribing, Phlebotomy, Medication) expertise will increase the value of your CV and open you up to multiple job sectors. Certificates Certificate of completion Digital certificate - Included

Search By Location
- Medicine and Nursing Courses in London
- Medicine and Nursing Courses in Birmingham
- Medicine and Nursing Courses in Glasgow
- Medicine and Nursing Courses in Liverpool
- Medicine and Nursing Courses in Bristol
- Medicine and Nursing Courses in Manchester
- Medicine and Nursing Courses in Sheffield
- Medicine and Nursing Courses in Leeds
- Medicine and Nursing Courses in Edinburgh
- Medicine and Nursing Courses in Leicester
- Medicine and Nursing Courses in Coventry
- Medicine and Nursing Courses in Bradford
- Medicine and Nursing Courses in Cardiff
- Medicine and Nursing Courses in Belfast
- Medicine and Nursing Courses in Nottingham