- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
19859 Courses
Data Protection (GDPR) Foundation Certificate
By Computer Law Training
This Foundation Certificate is an internationally recognised qualification, endorsed by TQUK, which is regulated by Ofqual, a UK Government department.

Celtic SMR are once again joining forces with ASA Laser to bring the hugely popular ‘Introduction to Therapeutic Laser’ workshops* to anyone interested in adding MLS® laser therapy to their clinic. These workshops are perfect for anyone wanting to learn more about what MLS® laser is, how it works and how to use it to treat a range of commonly seen MSK pathologies and wounds. Discover how to treat patients effectively and integrate laser seamlessly into your practice. Led by experts from ASA Laser, Salvatore Germano and Roberto Terruzzi and supported by UK MLS® laser trainer, this workshop will cover everything you need to know to start your laser journey in the best possible way. Learn the science behind MLS® laser therapy Learn how to use laser safely Discover how to achieve the best results for your patients Get hands on with MLS® Laser Opportunity to ask questions Got any questions about this workshop? Email sales@celticsmr.co.uk for help. *Please note these workshops are limited to 2 spaces per clinic.

Celtic SMR are once again joining forces with ASA Laser to bring the hugely popular ‘Introduction to Therapeutic Laser’ workshops* to anyone interested in adding MLS® laser therapy to their clinic. These workshops are perfect for anyone wanting to learn more about what MLS® laser is, how it works and how to use it to treat a range of commonly seen MSK pathologies and wounds. Discover how to treat patients effectively and integrate laser seamlessly into your practice. Led by experts from ASA Laser, Salvatore Germano and Roberto Terruzzi and supported by UK MLS® laser trainer, this workshop will cover everything you need to know to start your laser journey in the best possible way. Learn the science behind MLS® laser therapy Learn how to use laser safely Discover how to achieve the best results for your patients Get hands on with MLS® Laser Opportunity to ask questions Got any questions about this workshop? Email sales@celticsmr.co.uk for help. *Please note these workshops are limited to 2 spaces per clinic.

Basic ABG interpretation ABG interpretation training Introduction to ABG analysis Arterial blood gas interpretation Healthcare professional ABG course Acid-base balance training ABG parameters explanation Respiratory and metabolic disturbances Hands-on ABG practice Clinical applications of ABG interpretation ABG training for nurses/physicians/respiratory therapists ABG interpretation certification CPD accredited ABG course Practical ABG learning

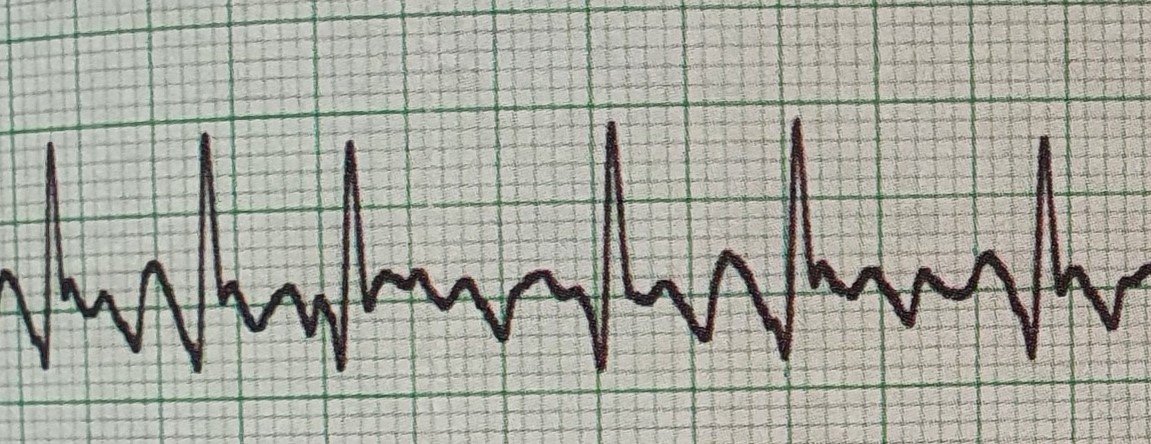
Basic ECG interpretation ECG basics for beginners ECG course for healthcare professionals ECG training for nurses Beginner ECG reading skills Introduction to ECG interpretation Understanding ECG rhythms Identifying common ECG abnormalities ECG strip reading practice ECG lead placement ECG graph paper essentials Interpreting normal sinus rhythms Recognizing cardiac arrhythmias Practical ECG exercises Hands-on ECG interpretation Expert instructors in ECG training CPD accredited ECG course 7 hours toward nursing revalidation Healthcare professional ECG certification Real-world ECG scenarios
A single day knife making course is a great way to make yourself an item that will last a lifetime, on this one day course we forge a small knife with a simple ash handle. A knife is one of the oldest tools ever made by humanity and it is one that invokes many emotions to people when they see and use it for a productive task. When doing a knife making course here at Bearded Pig Forge we will discuss the fine details of a knife and how they are made as well as the history of forging them. On the course you will learn the basics of forging a blade and heat treating them in order to get the best results from the high carbon steel, we will also be learning how to fit and shape handles by using a burn through technique to attach the blade to the handle. Courses are £165 Per person per day. I run a strict cancellation policy. If you cannot attend the date you have booked please contact me within three weeks and I will rebook your course for free, If you contact me after this deadline you will lose your place. Refunds are not available. No under 18s If you have a physical gift voucher it must be presented on the first day of a course.

Celtic SMR are once again joining forces with ASA Laser to bring the hugely popular ‘Introduction to Therapeutic Laser’ workshops* to anyone interested in adding MLS® laser therapy to their clinic. These workshops are perfect for anyone wanting to learn more about what MLS® laser is, how it works and how to use it to treat a range of commonly seen MSK pathologies and wounds. Discover how to treat patients effectively and integrate laser seamlessly into your practice. Led by experts from ASA Laser, Salvatore Germano and Roberto Terruzzi and supported by UK MLS® laser trainer, this workshop will cover everything you need to know to start your laser journey in the best possible way. Learn the science behind MLS® laser therapy Learn how to use laser safely Discover how to achieve the best results for your patients Get hands on with MLS® Laser Opportunity to ask questions Got any questions about this workshop? Email sales@celticsmr.co.uk for help. *Please note these workshops are limited to 2 spaces per clinic.

Food Safety, Education and Social Care Training Courses What You Need To Know About Food Allergies CPD Accredited, Interactive Short Course 2 hr session Do you serve food, or have people with known allergies in your school or on your team? In this webinar we will explain what food allergies are, and why it is vital for you to understand, for the health and safety, and indeed life, of the person involved Course Contents: What is a food allergy The 14 Allergens The Immune System Consequences of Food Allergies The importance of good food hygiene Benefits of this Short Course: More than 20% of the population in industrialized countries suffer from food intolerance or food allergy About two million people live with a diagnosed food allergy in the UK, and 32 million in the US 600,000 people in the UK have coeliac disease This course will teach you the importance of ensuring food is safe to eat for all, without causing significant pain or even death
An introduction course is a great way to learn about the amazing craft of blacksmithing, the course will teach you the basics of forging and making decorative work for your home and you will have something you have made to take home with you and enjoy. On the course we will make a twisted and scrolled fire poker and a hook to hang it, if we have time we may also make another small item such as a bottle opener or key ring Courses are £150 Per person per day I run a strict cancellation policy. If you cannot attend the date you have booked please contact me within three weeks and I will rebook your course for free, If you contact me after this deadline you will lose your place. Refunds are not available. No under 18s If you have a physical gift voucher it must be presented on the first day of a course. *Gift vouchers are valid for one year from date of purchase

Are you looking to enter the dynamic world of real estate? Our course is designed to equip you with the knowledge and tools you need to communicate effectively with real estate professionals and develop key skills in real estate investment strategy and analytics. At the end of the course, you'll be able to read and interpret real estate market reports, and have a firm grasp of how iconic buildings, cities, and companies fit into the overall picture of the real estate sector. On this course, you will… Become familiar with the players, structure, general terminology and overall needs of Real Estate. Learn what is Real Estate and why it is different from other asset classes Get to grips with the overall size and structure of the UK Real Estate Market Learn and analyse the links between the different parts of the property market Understand who works in the Real Estate Market, their qualifications and their job descriptions Recognise how and when to use basic real estate concepts: Rent, Value, Yield, Risk and Return, etc… Learn how to read a real estate market report Understand how current affairs, politics and economics affects Real Estate Investment Use household names and iconic companies, cities and buildings to help consolidate your appreciation of this exciting sector Who will benefit from this course: Graduates or undergraduates studying economics, finance. Professionals working in Marketing or Accounting teams within Real Estate firms. APC students. Anyone interested in Real Estate. School leavers/A-Level Students looking to gain an understanding of Real Estate. Non cognate students who wish to transfer into Real Estate/Finance careers. Course Outline Module 1: What is and why buy Real Estate? The property Market The Size and Structure of the UK property market The impact of Real Estate in the Economy Module 2: The Real Estate Market System The Space Market The Asset Market The Development Market Module 3: How to value Real Estate An Introduction to Financial Mathematics The difference between Price, Value and Worth Property Yield Conventional Valuation Methods Module 4: How to read a Real Estate Market Report Property Market Indicators: Stock Indicators Property Market Indicators: Investment Indicators Module 5: Who works in Real Estate? The build Environment by Cobalt Recruitment Rea; Estate Agents Examples of Real Estate Market Agents CVs Real Estate Network

Search By Location
- Introduction to Plant Science: Essential Concepts Courses in London
- Introduction to Plant Science: Essential Concepts Courses in Birmingham
- Introduction to Plant Science: Essential Concepts Courses in Glasgow
- Introduction to Plant Science: Essential Concepts Courses in Liverpool
- Introduction to Plant Science: Essential Concepts Courses in Bristol
- Introduction to Plant Science: Essential Concepts Courses in Manchester
- Introduction to Plant Science: Essential Concepts Courses in Sheffield
- Introduction to Plant Science: Essential Concepts Courses in Leeds
- Introduction to Plant Science: Essential Concepts Courses in Edinburgh
- Introduction to Plant Science: Essential Concepts Courses in Leicester
- Introduction to Plant Science: Essential Concepts Courses in Coventry
- Introduction to Plant Science: Essential Concepts Courses in Bradford
- Introduction to Plant Science: Essential Concepts Courses in Cardiff
- Introduction to Plant Science: Essential Concepts Courses in Belfast
- Introduction to Plant Science: Essential Concepts Courses in Nottingham