- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Courses matching "Clinical Research Administration: Protocols and Ethics"
Show all 91Explore the intricacies of Clinical Research Administration with our comprehensive course on Protocols and Ethics. Gain expertise in trial design, ethical considerations, regulatory compliance, and more. Prepare for a future in clinical research with practical insights and knowledge. Enroll now for a rewarding journey in advancing healthcare through responsible and ethical clinical trials.

Junior Clinical Researcher STAR Programme
By European Centre for Clinical Research Training (ECCRT)
How to increase your odds of finding a job in Clinical Research?This STAR Programme will provide the capabilities you need to become a Junior Clinical Researcher. Most employers will require practical experience on the job before considering your application. At ECCRT, we realise that this situation limits the inflow of young, intelligent, and motivated people. To further develop your potential, this STAR Programme combines a series of training and practical internships during a period of one year.

Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

Clinical Research Training for Junior CRAs
By European Centre for Clinical Research Training (ECCRT)
Reasons to attendThis Junior CRA training will train you in the basic, yet crucial areas within clinical monitoring. All typical tasks of a Clinical Research Associate (CRA) will become clear to you, from selecting the investigators until study site close-out, with a great focus on the “monitoring practice” and an introduction on the impact of digitalization. What's included? Documents and materials related to this course are included Globally recognised certificates awarded after test completion This course has been granted PharmaTrain Recognition

Clinical Project Management – Blended
By European Centre for Clinical Research Training (ECCRT)
Reasons to attendHow to ensure a successful clinical trial within timelines and budget? This Clinical Project Management training is a blended course (eLearning + Classroom or Webinars) designed to introduce the ins and outs of managing clinical research projects. The clinical study setting allows you to implement this knowledge immediately within your research projects.What's included? Documents and materials related to this course are included Globally recognised certificates awarded after test completion This course has been granted PharmaTrain Recognition

***24 Hour Limited Time Flash Sale*** Clinical Research Associate Diploma - CPD Certified Admission Gifts FREE PDF & Hard Copy Certificate| PDF Transcripts| FREE Student ID| Assessment| Lifetime Access| Enrolment Letter Are you a professional feeling stuck in your career, struggling to keep up with the ever-changing demands of the industry? Or perhaps you're a beginner, unsure of where to start or how to break into your desired field. Whichever stage you're in, our exclusive Clinical Research Associate Diploma - CPD Certified Bundle provides unique insights and tools that can help you achieve your goals. Designed to cater to the needs of both seasoned professionals and aspiring newcomers, our Clinical Research Associate Diploma - CPD Certified bundle is a comprehensive program that will equip you with the essential skills and knowledge you need to succeed. Whether you're looking to advance in your current role or embark on a new career journey, this bundle has everything you need to take your professional life to the next level. But that's not all. When you enrol in Clinical Research Associate Diploma - CPD Certified Online Training, you'll receive 25 CPD-Accredited PDF Certificates, Hard Copy Certificates, and our exclusive student ID card, all absolutely free. Courses Are Included In this Clinical Research Associate Diploma - CPD Certified Career Bundle: Course 01: Clinical Research Administration: Navigating the Healthcare Landscape Course 02: Research in Adult Care Course 03: Introduction to Medical Terminology Course 04: Anatomy and Physiology of Human Body Course 05: Introduction to Health Economics and Health Technology Assessment Course 06: Biochemistry Course 07: Genetics Course 08: Neurology Course 09: Pharmacology Course 10: Pharmacy Assistant and Technician Foundation Diploma Course 11: Mental Health and Psychiatry Training Course 12: Infection Prevention and Immunisation Course 13: Healthcare GDPR Training Course 14: Medical Secretary Diploma Course 15: Medical Receptionist Course - CPD Accredited Course 16: Medical Law Course 17: Medical Transcription: Audio Typist Course 18: Nurse Prescribing Diploma Course 19: Radiography Course 20: Strengthen Your Immune System Course 21: Medical Writing Course 22: Effective Communication Techniques for Teachers and Trainers Course 23: Public Health Course 24: Initial Training of Community Health Agents Course 25: Risk Assessment in Health & Social Care With Level 7 in Strategic Learning & Development, you'll embark on an immersive learning experience that combines interactive lessons with voice-over audio, ensuring that you can learn from anywhere in the world, at your own pace. And with 24/7 tutor support, you'll never feel alone in your journey, whether you're a seasoned professional or a beginner. Don't let this opportunity pass you by. Enrol in Clinical Research Associate Diploma - CPD Certified today and take the first step towards achieving your goals and dreams. Why buy this Level 7 in Strategic Learning & Development? Free CPD Accredited Certificate upon completion of Level 7 in Strategic Learning & Development Get a free student ID card with Level 7 in Strategic Learning & Development Lifetime access to the Clinical Research Associate Diploma - CPD Certified course materials Get instant access to this Clinical Research Associate Diploma - CPD Certified course Learn Clinical Research Associate Diploma - CPD Certified from anywhere in the world 24/7 tutor support with the Clinical Research Associate Diploma - CPD Certified course. Start your learning journey straightaway with our Clinical Research Associate Diploma - CPD Certified Training! Clinical Research Associate Diploma - CPD Certified premium bundle consists of 25 precisely chosen courses on a wide range of topics essential for anyone looking to excel in this field. Each segment of the Clinical Research Associate Diploma - CPD Certified is meticulously designed to maximise learning and engagement, blending interactive content and audio-visual modules for a truly immersive experience. Certification You have to complete the assignment given at the end of the Clinical Research Associate Diploma - CPD Certified course. After passing the Clinical Research Associate Diploma - CPD Certified exam You will be entitled to claim a PDF & Hardcopy certificate accredited by CPD Quality standards completely free. CPD 250 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Clinical Research Associate Diploma - CPD Certified course is ideal for: Students seeking mastery in Level 7 in Strategic Learning & Development Professionals seeking to enhance Clinical Research Associate Diploma - CPD Certified skills Individuals looking for a Level 7 in Strategic Learning & Development-related career. Anyone passionate about Level 7 in Strategic Learning & Development Requirements This Clinical Research Associate Diploma - CPD Certified doesn't require prior experience and is suitable for diverse learners. Career path This Clinical Research Associate Diploma - CPD Certified bundle will allow you to kickstart or take your career in the related sector to the next stage. Certificates CPD Accredited Hard Copy Certificate Hard copy certificate - Included If you are an international student, you will be required to pay an additional fee of 10 GBP for international delivery, and 4.99 GBP for delivery within the UK, for each certificate CPD Accredited Digital Certificate Digital certificate - Included

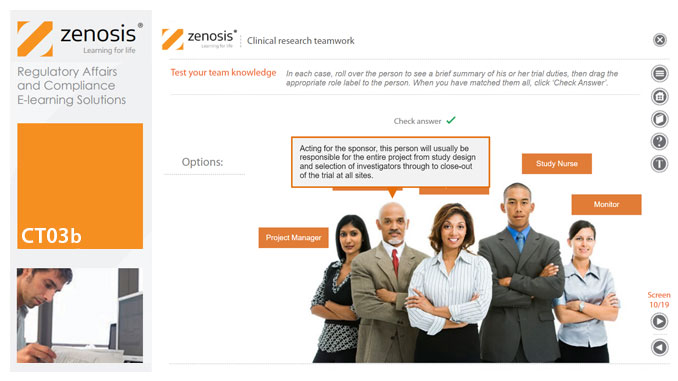
CT03b - Clinical research teamwork
By Zenosis
A clinical trial, particularly a late-phase commercial study, is a major project requiring collaboration between the sponsor and staff or contractor, on the one hand, and the clinical investigator(s) and other healthcare professionals on the other. Good communication among all parties is essential. In this short course we introduce the major roles in a typical clinical research project and outline their duties.
Clinical Research Training for Senior CRAs
By European Centre for Clinical Research Training (ECCRT)
Reasons to attendDo you want to refresh and improve your CRA skills? Do you need some inspiration to boost your clinical trial monitoring techniques and approach? This CRA Training provides for experienced monitors the knowledge and advanced skills to deal with more complex clinical trials and site management issues. This training, supported by eLearning, provides the best outcome allowing you to learn at your own pace. Increase your odds of becoming a senior CRA with this training. What's included? Documents and materials related to this course are included Globally recognised certificates awarded after test completion This course has been granted PharmaTrain Recognition

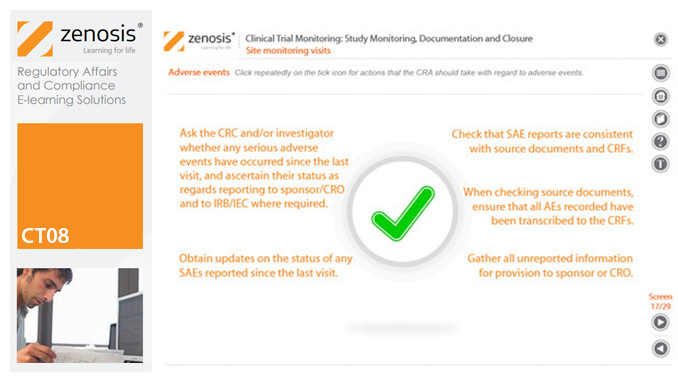
CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
By Zenosis
The sponsor of a clinical trial must arrange for it to be monitored throughout its duration to ensure that the rights and wellbeing of subjects are protected, the trial data are accurate, complete and verified from source documents, and the conduct of the trial complies with the study protocol, Good Clinical Practice and regulatory requirements. In this module we describe how a Clinical Research Associate (CRA) monitors an ongoing trial to its conclusion.
Search By Location
- Clinical Research Administration: Protocols and Ethics Courses in London
- Clinical Research Administration: Protocols and Ethics Courses in Birmingham
- Clinical Research Administration: Protocols and Ethics Courses in Glasgow
- Clinical Research Administration: Protocols and Ethics Courses in Liverpool
- Clinical Research Administration: Protocols and Ethics Courses in Bristol
- Clinical Research Administration: Protocols and Ethics Courses in Manchester
- Clinical Research Administration: Protocols and Ethics Courses in Sheffield
- Clinical Research Administration: Protocols and Ethics Courses in Leeds
- Clinical Research Administration: Protocols and Ethics Courses in Edinburgh
- Clinical Research Administration: Protocols and Ethics Courses in Leicester
- Clinical Research Administration: Protocols and Ethics Courses in Coventry
- Clinical Research Administration: Protocols and Ethics Courses in Bradford
- Clinical Research Administration: Protocols and Ethics Courses in Cardiff
- Clinical Research Administration: Protocols and Ethics Courses in Belfast
- Clinical Research Administration: Protocols and Ethics Courses in Nottingham

