- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
3097 Courses
**10 FREE QLS Endorsed Certificates and Included with Lifetime Access** People are the heartbeat of every organisation—and managing people takes more than just a smile and a spreadsheet. This Human Resources Management Diploma Level 4 (QLS Endorsed) is built for those who want to understand what truly keeps a team running smoothly. From hiring to handling disputes, building better staff engagement to understanding workplace laws—this course digs into the details HR professionals need to know. If your idea of HR is just holiday requests and interviews, think again. This goes deeper—minus the jargon and fluff.The course is structured to give you solid HR knowledge from all sides, whether it’s recruitment, performance reviews, or handling sticky situations with professionalism. It’s designed for those aiming to lead or support HR functions without needing years of prior experience. Whether you're working in admin, team leadership, or looking to explore a new direction in people management—this course will give you the right kind of knowledge, in the right kind of way. Fully online, flexible, and informative without putting you to sleep. Human Resources Management - QLS Endorsed Bundle Includes the following Courses Course 01: Diploma in Human Resources Management at QLS Level 4 Course 02: Diploma in Change Management at QLS Level 5 Course 03: Certificate in Bereavement and Grief Counselling at QLS Level 3 Course 04: Diploma in Corporate Compliance and Risk Management at QLS Level 3 Course 05: Certificate in Office Admin and Organisation Skills at QLS Level 3 Course 06: Certificate in Public Relations at QLS Level 3 Course 07: Award in Event Management at QLS Level 2 Course 08: Diploma in Career Coaching at QLS Level 5 Course 09: Diploma in Security Management at QLS Level 5 Course 10: Advanced Diploma in Project Management at QLS Level 7 Learning Outcomes Develop event planning expertise for seamless execution. Acquire adept office administration and organisational skills. Master corporate compliance and risk management strategies. Hone public relations techniques for effective communication. Attain advanced proficiency in human resources management. Navigate complex corporate governance principles and practices. Develop security management skills for a secure work environment. Cultivate grief counselling capabilities with sensitivity and empathy. Gain strategic insight into project management at an advanced level. Excel in the integration of diverse skills across various disciplines. Key Features 10 FREE QLS Endorsed Certificate Fully online, interactive course Self-paced learning and laptop, tablet, smartphone-friendly 24/7 Learning Assistance Discounts on bulk purchases Behind every successful sales team is not just a string of impressive numbers, but a thriving workplace culture crafted by our dedicated Human Resources team. In this bundle, we explore how the human element, championed by HR, is a key ingredient in our journey to success. This curated collection of courses spans a range of essential disciplines, empowering you with versatile skills applicable across diverse industries. Dive into the intricacies of Event Management and HR Management, honing your organisational prowess. Explore the nuances of Bereavement and Grief Counselling, fostering empathy and support. Elevate your strategic thinking with Diplomas in Corporate Compliance, Risk Management, and Corporate Governance, ensuring you're equipped to navigate complex business landscapes. Cultivate essential administrative finesse with Certificates in Office Admin, Organisation Skills, and Public Relations. Progress through the tiers, mastering Human Resources Management and Security Management, culminating in an Advanced Diploma in Project Management. This multifaceted bundle is your gateway to a well-rounded skill set, providing a springboard for success in various professional arenas. By doing this bundle you will gain the ultimate understanding in strategic planning, organisational leadership, crisis management, and interpersonal communication. Develop expertise in compliance, risk mitigation, and project execution. Sharpen administrative skills for seamless office operations and public relations finesse. Master the intricacies of human resources and security management, equipping yourself for diverse roles in the professional landscape. This bundle is designed to nurture well-rounded professionals with a holistic understanding of critical business functions. Certificate Once you've successfully completed your course, you will immediately be sent a CPD Accredited PDF certificate. Also, you can have your printed certificate delivered by post (shipping cost £3.99). After successfully completing the assignment, learners will be able to order FREE QLS Endorsed certificate for Each Courses. CPD 60 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Aspiring Health and Safety Professionals Managers and Supervisors Mental Health Care Workers Childcare and Education Professionals Individuals in Substance Control Roles Psychology Enthusiasts Art Therapy Practitioners Those Pursuing Career Advancement Career path Health and Safety Manager Mental Health Care Coordinator Child Safeguarding Officer Substance Control Specialist Psychology Research Assistant Art Therapist Certificates Digital certificate Digital certificate - Included Hard copy certificate Hard copy certificate - Included Hardcopy Certificate (UK Delivery): For those who wish to have a physical token of their achievement, we offer a high-quality, printed certificate. This hardcopy certificate is also provided free of charge. However, please note that delivery fees apply. If your shipping address is within the United Kingdom, the delivery fee will be only £3.99. Hardcopy Certificate (International Delivery): For all international addresses outside of the United Kingdom, the delivery fee for a hardcopy certificate will be only £10. QLS Endorsed Certificate Hard copy certificate - Included

Perfect 3 Days Linux Course
By Packt
Linux administration and command line

Get Noticed By Recruiters in this Hiring Season by Developing Your Skills! Make a strong base as a teaching assistant and bright enough your education and training proficiency through a certified Level 4 Certificate for Higher Level Teaching Assistants (RQF) Qualifications. This Level 4 Certificate for Higher Level Teaching Assistants (RQF) course is awarded by an established Ofqual-regulated Awarding Organisation named Focus Awards Limited, which ensures you've demonstrated the requisite competency and this competence is acknowledged. It will provide you with essential skills that allow you to start or succeed in a successful teaching career. This most engaging Level 4 Certificate for Higher Level Teaching Assistants (RQF) course assures both professional and certified qualifications for you. With the special course materials that are online and accessible 24/7 from anywhere in the globe, you will be able to master the A-Z of education and training. The contents are designed to equip you with a fundamental and advanced understanding of all aspects of teaching and management, such as communication, relationship building, decision-making processes, innovation, and much more! It will also provide you with a prestigious acknowledgement that allows you to start or succeed in a successful teaching career. Enrol now and be a top-notch teaching professional!!! Why is this Level 4 Certificate for Higher Level Teaching Assistants (RQF)Perfect for You? Change Your Career: Join today and make a life-changing decision, get all the essential knowledge to transform your career. Advance Your Career: With our Regulated qualification, you can move up to the next level in your organisation with expertise. Upgrade Your Skills: Add value to your current educational profile and gain the skill sets to compete in your job role. Enhance Your Credibility: The Level 4 Certificate for Higher Level Teaching Assistants (RQF) Course is Ofqual, UK Govt. Regulated and Awarded by Focus Awards which adds value to your educational profile Cost Efficient: In most cases, British qualification starts from £3000 per annum, but with Apex Learning, you enjoy savings of up to 80% on your educational investment. 100% Money-Back Guarantee: We are confident about our course quality and want to provide the best service to our invaluable learners. That's why we provide 14 days Unconditional Full Money-Back Guarantee with this course to ensure a hassle-free purchase. Benefits you will gain from this Level 4 Certificate for Higher Level Teaching Assistants (RQF) course : Premium quality, intensive e-learning course materials Find a well-defined website for teaching 24/7 teacher assistance Step-by-step guidelines Budget-friendly price Earned recognition from the Uk's top awarding bodies Study in a user-friendly, advanced student portal Convenient and Flexible time limit Qualification Summary Qualification type: Focus Awards Level 4 Award Qualification title: Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) Accreditation status: Accredited Level: 4 Guided Learning Hours (GLH): 87 Total Qualification Time (TQT): 360 Qualification number (QN): 601/8533/8 Course Curriculum Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) course comprises seven mandatory units. These are as follows: Awareness of special educational needs Child development and welfare Curriculum planning, delivery and assessment Developing skills to promote positive working relationships Promoting positive behaviour in children and young people Providing support for individual learners and small groups Understand the Higher Level Teaching Assistant role ****Qualification Curriculum**** **Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF)** Unit 1: Understand the Higher Level Teaching Assistant role Unit 2: Child development and welfare Unit 3: Awareness of special educational needs Unit 4: Promoting Positive Behaviour in Children and Young People Unit 5: Providing support for individual learners and small groups Unit 6: Developing skills to promote positive working relationships Unit 7: Curriculum planning, delivery and assessment Learning Outcomes What skills will I gain from this Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) Course? Inclusive Teaching and Learning Teaching, Learning and Assessment Approaches Providing opportunities for English, Maths, ICT and Wider Skills Theories of Learning Communication Induction, Icebreakers and Ground Rules Creating a Scheme of Work Devising an Inclusive Teaching and Learning Plan (Session Plan) Understand the Higher Level Teaching Assistant role Self-Evaluation and Continuing Professional Development Learning Duration Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) Total Qualification Time It is an estimate of the total amount of time anticipated that a learner would spend to demonstrate mastery of all learning outcomes to achieve the award of the qualification. The whole qualification time is defined as GLH and an estimate of the time a learner will devote to preparation, study, and assessment. It does not underlie supervision by a lecturer, supervisor, or tutor. In the case of qualification, the credit value is defined by TQT, and one credit corresponds to ten hours of learning. Guided Learning Hours for this qualification is 87. Total Qualification Time for this qualification is 360 hours. The total credit value for this qualification is 36 Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) Guided Learning Hours These hours comprise all real-time contact time, hours of lectures or tutorial or supervision of a learner, tutor, trainer or other education providers. Progression Level 4 Certificate in Education and Training Level 5 Diploma in Education and Training Level 3 Award in Understanding the Principles and Practices of Assessment Level 3 Award in Assessing Competence in the Work Environment Level 3 Award in Assessing Vocational Related Achievement Level 3 Certificate in Assessing Vocational Achievement Level 4 Award in Understanding the Internal Quality Assurance of Assessment Processes and Practice Level 4 Award in the Internal Quality Assurance of Assessment Processes and Practice Level 4 Certificate in Leading the Internal Quality Assurance of Assessment Processes and Practice Level 4 Award in Learning and Development Level 4 Diploma in Learning and Development Method of Assessment: Unit 1: In Unit 1, you need to complete a variety of writing assignments Unit 2: In Unit 2, there should be an assessment taken in the workplace, primarily evaluating learners' work, professional discussions with audio-video evidence Unit 3: In Unit 3, you will be demonstrated through your delivered work items and audio-video evidence after evaluating learners in a training context. Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) Video Assessment You have to plan many things, such as observation, questioning/ professional discussion, and inspection of supporting work products evidence. Detailed assignment instructions will be made available to you in the 'Guidance for Achieving the Unit' section of your learning portal with a careful and clear explanation. You have to submit all your assignments via the online portal Certification Successful candidates will be awarded a Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) from Focus Awards Limited. Who is this course for? You will have the ability to accomplish a lot of things with this course certificate. Here are just a few examples: Primary School Teacher Secondary School Teacher Private Tutor Freelance Teacher/ Trainer Consultant Job hunters & School leavers Educational Psychologist Office clerk & Administration Assistant College or University Student Requirements The Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) course does not have any specific entry requirements for students. However, you must be at least 16 years old or above to register and undergo an initial assessment. Since it is a level 3 qualification, you must pass the minimum 1st level of the literacy assessment Career path You will have the ability to accomplish a lot of things with this course certificate. Here are just a few examples: Primary School Teacher Secondary School Teacher Private Tutor Freelance Teacher/ Trainer Consultant Job hunters & School leavers Educational Psychologist Office clerk & Administration Assistant College or University Student

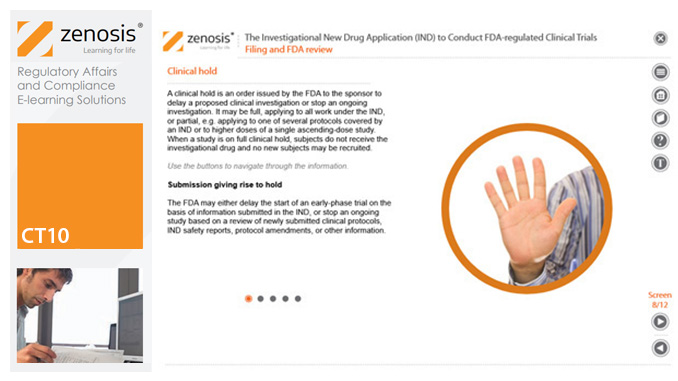
CT10: The Investigational New Drug Application (IND) to Conduct FDA-regulated Clinical Trials
By Zenosis
An Investigational New Drug Application (IND) is a submission to the US Food and Drug Administration (FDA) for permission to conduct a clinical trial of a medicinal product. This module describes regulatory requirements that sponsors or sponsor-investigators must meet for successful compilation, filing and maintenance of INDs. The IND and its role are defined, and the contexts in which it is required are specified.
Tired of searching and accumulating all the relevant courses for this specific field? It takes a substantial amount of your time and, more importantly, costs you a fortune! Well, we have just come up with the ultimate solution for you by giving this all-inclusive Office Skills and Administration Essentials mega bundle. This 30 courses mega bundle keeps you up-to-date in this field and takes you one step ahead of others. Keeping in mind the latest advancements in this ever-emerging sector, the Office Skills and Administration Essentials bundle covers all the state-of-the-art tools and techniques required to become a competent worker in this area. You will encounter specific courses relevant to the sector. We take you from the most introductory fundamentals to advance knowledge in a step-by-step manner. In addition, the Office Skills and Administration Essentials bundle also consists of courses related to some soft skills that you will need to succeed in any industry or job sector. This Office Skills and Administration Essentials Bundle consists of the following premium courses: Course 01: Office Skills Course 02: Admin, Secretarial & PA Course 03: Performance Management Course 04: Team Management Advanced Training Course 05: Business Etiquette & Professionalism Course 06: Information Management for Beginners Course 07: Equality, Diversity and Discrimination Course 08: Coaching & Mentoring Diploma Course 09: Presentation Skills - Present like a Pro Course 10: Workplace Confidentiality Basics Course 11: Critical Thinking & Problem Solving Course 12: Email Writing Training Course 13: Public Speaking Course Course 14: Corporate Leadership Training Course 15: Corporate Risk And Crisis Management Course 16: Cross-Cultural Awareness Course Course 17: Meeting Management Online Course 18: Minute Taking Masterclass Course 19: Document Control and Management Course 20: Touch Typing Training Course 21: Line Management Diploma Course 22: Facilities Management Course 23: UK Employment Law Course 24: Organisational Chaos Management Course 25: Basic Communication Skills for Business Moreover, this bundles include 5 career-focused courses: Course 01: Career Development Plan Fundamentals Course 02: CV Writing and Job Searching Course 03: Interview Skills: Ace the Interview Course 04: Video Job Interview for Job Seekers Course 05: Create a Professional LinkedIn Profile Our cutting-edge learning package offers top-notch digital aid and first-rate tutor support. You will acquire the crucial hard and soft skills needed for career advancement because this bundle has been thoroughly examined and is career-friendly. So don't overthink! Enrol today. Learning Outcomes This unique Office Skills and Administration Essentials mega bundle will help you to- Quench your thirst for knowledge Be up-to-date about the latest advancements Achieve your dream career goal in this sector Know the applicable rules and regulations needed for a professional in this area Acquire some valuable knowledge related to Office Skills and Administration Essentials to uplift your morale The bundle incorporates basic to advanced level skills to shed some light on your way and boost your career. Hence, you can strengthen your expertise and essential knowledge, which will assist you in reaching your goal. Moreover, you can learn from any place in your own time without travelling for classes. Certificate: PDF Certificate: Free for all 30 courses Hard Copy Certificate: Free (For The Title Course: Previously it was £10) CPD 300 CPD hours / points Accredited by CPD Quality Standards Who is this course for? The Office Skills and Administration Essentials bundle is designed to assist anyone with a curious mind, anyone looking to boost their CVs or individuals looking to upgrade their career to the next level can also benefit from the learning materials. Requirements The courses in this bundle has been designed to be fully compatible with tablets and smartphones. Career path This Office Skills and Administration Essentials bundle will give you an edge over other competitors and will open the doors for you to a plethora of career opportunities. Certificates Certificate of completion Digital certificate - Included Certificate of completion Hard copy certificate - Included You will get the Hard Copy certificate for the title course (Office Skills) absolutely Free! Other Hard Copy certificates are available for £10 each. Please Note: The delivery charge inside the UK is £3.99, and the international students must pay a £9.99 shipping cost.

Microsoft Office Skills (MS Excel, Word, PowerPoint, Access), Administration & IT Training
4.8(9)By Skill Up
All-in-one Package| CPD Certified| 35 Courses Training| 350 CPD Points| Free PDF+ Transcript Certificate| Lifetime Access

Certificate in Fund Administration
By International Compliance Association
This qualification from our partner organisation CLTI looks at the practical areas of fund structures and strategies, accounting and valuation, taxation, and regulation for those working in fund administration or support. It will enable you to develop an understanding of the funds industry and some of the key roles that support fund operations, and consider the different fund structures, strategies for investment and regulatory obligations. You will be able to: understand the fundamental workings of the fund industry, including relevant terminology, and the roles and responsibilities of the different teams supporting a fund. identify and understand different fund structures and the documents relating to them. explain the differences between, various fund strategies. be familiar with key terminology and processes in relation to accounting and valuations, and be able to carry out straightforward calculations. understand the basic principles of the taxation of funds, including VAT and transfer pricing, and be able to carry out straightforward calculations. understand the key regulation affecting fund administrators and the obligations placed upon the fund administrator by company law and AML provisions. This qualification covers the following topics: The Fund Industry Fund structures Fund strategies Fund accounting and fund valuations Taxation Regulation

=> Updated Course <= Get Noticed By Recruiters in this Hiring Season by Developing Your Skills! Make a strong base as a teaching assistant and bright enough your education and training proficiency through a certified Level 4 Certificate for Higher Level Teaching Assistants (RQF) Qualifications. This Level 4 Certificate for Higher Level Teaching Assistants (RQF) course is awarded by an established Ofqual-regulated Awarding Organisation named Focus Awards Limited, which ensures you've demonstrated the requisite competency and this competence is acknowledged. It will provide you with essential skills that allow you to start or succeed in a successful teaching career. This most engaging Level 4 Certificate for Higher Level Teaching Assistants (RQF) course assures both professional and certified qualifications for you. With the special course materials that are online and accessible 24/7 from anywhere in the globe, you will be able to master the A-Z of education and training. The contents are designed to equip you with a fundamental and advanced understanding of all aspects of teaching and management, such as communication, relationship building, decision-making processes, innovation, and much more! It will also provide you with a prestigious acknowledgement that allows you to start or succeed in a successful teaching career. Enrol now and be the top-notch teaching professional!!! Why is this Level 4 Certificate for Higher Level Teaching Assistants (RQF)Perfect for You? Change Your Career: Join today and make a life-changing decision, get all the essential knowledge to transform your career. Advance Your Career: With our Regulated qualification, you can move up to the next level in your organisation with expertise. Upgrade Your Skills: Add value to your current educational profile and gain the skill sets to compete in your job role. Enhance Your Credibility: The Level 4 Certificate for Higher Level Teaching Assistants (RQF) Course is Ofqual, UK Govt. Regulated and Awarded by Focus Awards which adds value to your educational profile Cost Efficient: In most cases, British qualification starts from £3000 per annum, but with Apex Learning, you enjoy savings of up to 80% on your educational investment. 100% Money-Back Guarantee: We are confident about our course quality and want to provide the best service to our invaluable learners. That's why we provide 14 days Unconditional Full Money-Back Guarantee with this course to ensure a hassle-free purchase. Benefits you will gain from this Level 4 Certificate for Higher Level Teaching Assistants (RQF) course : Premium quality, intensive e-learning course materials Find a well-defined website for teaching 24/7 teacher assistance Step-by-step guidelines Budget-friendly price Earned recognition from the Uk's top awarding bodies Study in a user-friendly, advanced student portal Convenient and Flexible time limit Qualification Summary Qualification type: Focus Awards Level 4 Award Qualification title: Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) Accreditation status: Accredited Level: 4 Guided Learning Hours (GLH): 87 Total Qualification Time (TQT): 360 Qualification number (QN): 601/8533/8 Course Curriculum Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) course comprises seven mandatory units. These are as follows: Awareness of special educational needs Child development and welfare Curriculum planning, delivery and assessment Developing skills to promote positive working relationships Promoting positive behaviour in children and young people Providing support for individual learners and small groups Understand the Higher Level Teaching Assistant role ****Qualification Curriculum**** **Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF)** Unit 1: Understand the Higher Level Teaching Assistant role Unit 2: Child development and welfare Unit 3: Awareness of special educational needs Unit 4: Promoting Positive Behaviour in Children and Young People Unit 5: Providing support for individual learners and small groups Unit 6: Developing skills to promote positive working relationships Unit 7: Curriculum planning, delivery and assessment ***Curriculum of The FREE Courses*** **Teaching Assistant** Module 01: Overview Module 02: Professional Developments for a TA Module 03: Working with the School and Its Policies Module 04: Discussion on Curriculum, Assessment and Monitoring Module 05: Providing the Best Learning Environment Module 06: Learn to Implement ICT, Literacy and Numeracy Development Processes as a TA Module 07: Child Development and the SEN Support Module 08: Understanding Behaviour & Relationships as a TA Module 09: Experienced TAs Maintain Strong Communication with the Students Module 10: Career Prospects for TAs in the UK ** SEN Teaching ** Module 01: Special Education Needs (SEN) An Overview Module 02: SEN Support Module 03: High-Quality Teaching for Pupils with SEN Module 04: SEN Teaching Methodologies Module 05: Communication and Interaction Module 06: Cognition and Learning Module 07: Social, Emotional and Mental Health Difficulties Module 08: Sensory or Physical Needs Module 09: Working in Partnership **EYFS Teaching - Level 3** Module 01: Introduction to EYFS Module 02: Importance of Early Years in Development Module 03: EYFS Teaching Techniques Module 04: Working as an EYFS Teacher Module 05: Promoting Learning and Development Module 06: Children Having Special Education Needs or Disabilities Module 07: Parents' Guide to the Early Years Foundation Stage Module 08: Welfare Requirements Module 09: The EYFS Educational Philosophies and Privileges Module 10: Registration, Inspection, and Quality Improvement Module 11: EYFS Framework in 2021 Learning Outcomes What skills will I gain from this Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) Course? Inclusive Teaching and Learning Teaching, Learning and Assessment Approaches Providing opportunities for English, Maths, ICT and Wider Skills Theories of Learning Communication Induction, Icebreakers and Ground Rules Creating a Scheme of Work Devising an Inclusive Teaching and Learning Plan (Session Plan) Understand the Higher Level Teaching Assistant role Self-Evaluation and Continuing Professional Development Learning Duration Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) Total Qualification Time It is an estimate of the total amount of time anticipated that a learner would spend to demonstrate mastery of all learning outcomes to achieve the award of the qualification. The whole qualification time is defined as GLH and an estimate of the time a learner will devote to preparation, study, and assessment. It does not underlie supervision by a lecturer, supervisor, or tutor. In the case of qualification, the credit value is defined by TQT, and one credit corresponds to ten hours of learning. Guided Learning Hours for this qualification is 87. Total Qualification Time for this qualification is 360 hours. The total credit value for this qualification is 36 Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) Guided Learning Hours These hours comprise all real-time contact time, hours of lectures or tutorial or supervision of a learner, tutor, trainer or other education providers. Progression Level 4 Certificate in Education and Training Level 5 Diploma in Education and Training Level 3 Award in Understanding the Principles and Practices of Assessment Level 3 Award in Assessing Competence in the Work Environment Level 3 Award in Assessing Vocational Related Achievement Level 3 Certificate in Assessing Vocational Achievement Level 4 Award in Understanding the Internal Quality Assurance of Assessment Processes and Practice Level 4 Award in the Internal Quality Assurance of Assessment Processes and Practice Level 4 Certificate in Leading the Internal Quality Assurance of Assessment Processes and Practice Level 4 Award in Learning and Development Level 4 Diploma in Learning and Development Assessment The qualification assessments will be taken through tutor-marked assignments. Upon completing each unit, the learner must attempt a series of questions with comprehensive answers, which will be marked by the tutor. The tutor-marked tasks are required to be assessed by a professional as well. Thereupon, to ensure all the course assignments have met the standards properly by both the learners and assessors, the tasks are subjected to internal and external moderation. NB: At least 50 hours of work placement within a school is required to accomplish this assessment. The work setting can either be a primary, secondary or special school with pupils aged 5+ and studying Key Stage 1 or above. As a part of this placement, learners will require a member with occupational competence in supervising and signing the witness testimonies, to ensure they have gained the work-based learning outcomes of this programme properly. Method of Assessment: Unit 1: In Unit 1, you need to complete a variety of writing assignments Unit 2: In Unit 2, there should be an assessment taken in the workplace, primarily evaluating learners' work, professional discussions with audio-video evidence Unit 3: In Unit 3, you will be demonstrated through your delivered work items and audio-video evidence after evaluating learners in a training context. Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) Video Assessment You have to plan many things, such as observation, questioning/ professional discussion, and inspection of supporting work products evidence. Detailed assignment instructions will be made available to you in the 'Guidance for Achieving the Unit' section of your learning portal with a careful and clear explanation. You have to submit all your assignments via the online portal Certification Successful candidates will be awarded a Focus Awards Level 4 Certificate for Higher Level Teaching Assistants (RQF) from Focus Awards Limited. Requirements You must be at least 16 years old or above to register and undergo an initial assessment. Since it is level 3 qualification, you must pass the minimum 1st level of the literacy assessment NB: At least 50 hours of work placement within a school is required to accomplish this assessment. The job setting can either be a primary, secondary or special school with pupils aged 5+ and studying Key Stage 1 or above. As a part of this placement, learners will require a member with occupational competence in supervising and signing the witness testimonies, to ensure they have gained the work-based learning outcomes of this programme properly. Career path You will have the ability to accomplish a lot of things with this course certificate. Here are just a few examples: Primary School Teacher Secondary School Teacher Private Tutor Freelance Teacher/ Trainer Consultant Job hunters & School leavers Educational Psychologist Office clerk & Administration Assistant College or University Student

First Aid Trainer - QLS Endorsed Bundle
By Imperial Academy
10 QLS Endorsed Courses for First Aid Trainer | 10 Endorsed Certificates Included | Life Time Access

Search By Location
- Administration of Insulin Courses in London
- Administration of Insulin Courses in Birmingham
- Administration of Insulin Courses in Glasgow
- Administration of Insulin Courses in Liverpool
- Administration of Insulin Courses in Bristol
- Administration of Insulin Courses in Manchester
- Administration of Insulin Courses in Sheffield
- Administration of Insulin Courses in Leeds
- Administration of Insulin Courses in Edinburgh
- Administration of Insulin Courses in Leicester
- Administration of Insulin Courses in Coventry
- Administration of Insulin Courses in Bradford
- Administration of Insulin Courses in Cardiff
- Administration of Insulin Courses in Belfast
- Administration of Insulin Courses in Nottingham
