- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
43095 Courses
Microsoft Office 365 for End Users In-Company (now with live online classes)
By Microsoft Office Training
This 1 day course is designed for the end user who is using or will use Office 365. This course will provide delegates with the knowledge and skills to efficiently use Office 365 on a day-to-day basis. The course is designed with real world scenarios in mind. Delegates will learn how to use Outlook Online, Skype for Business, OneDrive for Business, SharePoint Online, and OneNote. At the end of this course delegates will be able to effectively navigate Office 365 and make use of all of the features of Office 365 Office 365 Overview Introducing Cloud Computing Identify and Outline the Component Products in Office 365 including Outlook Web App, Office Online Apps, OneDrive and Skype for Business Navigating around Office 365 Customising the Office 365 Nav Bar Updating your Profile in Office 365 Using the Outlook Online Application Overview of Outlook Online Working with Email and Folders Outlook People and IM Contacts Using the Calendar Shared Calendars Outlook Tasks in the Web App Setting Outlook Options, Signatures, Automatic Replies and Rules Using Skype for Business Overview of Skype for Business Viewing and Setting Presence Status Using Instant Messages in Business Understanding the Interactive Contact Card in Microsoft Office Applications Integration with Outlook Using Skype for Business for Online Presentations including Content Sharing, Polls and a Virtual Whiteboard Working with OneDrive for Business What is OneDrive for Business? Navigating around OneDrive Accessing Content in OneDrive Using the Office Online Apps Sharing Documents and Collaborating Connecting Microsoft Office to OneDrive Creating Office Documents and Saving Directly to OneDrive Using Groups and Delve Introduction to Groups Collaborating using Groups Getting to Content using Delve Requirements Requirements Before attending this course, students must have: Basic understanding of Microsoft Office Basic understanding of Microsoft Windows Operating systems

Level 3 Law Diploma with Employment & Labour Law
By Kingston Open College
Premium Bundle of all Time | Ofqual Regulation + ATHE Awards + CPD Accreditation | Assessment & Tutor Support Included

Certificate in Anti Money Laundering
By The Association of Governance, Risk & Compliance
Become a financial crime prevention superhero with LGCA’s help! Pick up everything you need to know to join a company’s AML function and start fighting crime with your newfound knowledge and skills. The Certificate in AML provides participants with a thorough understanding of what constitutes Money Laundering (ML) and how financial institutions should respond to increasingly complex attempts by criminal individuals and entities to process proceedings from illegal activities in a manner that enables them to enjoy such illegal proceedings.

NPORS Harness and Fall Arrest (N723)
By Dynamic Training and Assessments Ltd
NPORS Harness and Fall Arrest (N723)

NPORS Pallet - Stacker Truck (N008)
By Dynamic Training and Assessments Ltd
NPORS Pallet - Stacker Truck (N008)

NPORS Rough Terrain Lift Truck (N009)
By Dynamic Training and Assessments Ltd
NPORS Rough Terrain Lift Truck (N009)

NPORS Side Loader (N006)
By Dynamic Training and Assessments Ltd
NPORS Side Loader (N006)

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

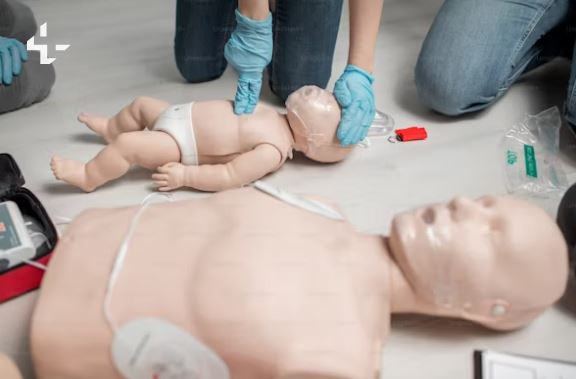
First Aid Trainer - Level 3 Emergency Paediatric First Aid Course - On-site Training
By Kitchen Tonic Training Company and Food Safety Consultants
This paediatric first aid course provides essential information related to the Level 3 Award in Emergency Paediatric First Aid (RQF). Participants will gain vital skills and knowledge necessary to respond effectively to emergencies involving infants and children, ensuring the safety and well-being of young individuals in critical situations.
Search By Location
- knowledge Courses in London
- knowledge Courses in Birmingham
- knowledge Courses in Glasgow
- knowledge Courses in Liverpool
- knowledge Courses in Bristol
- knowledge Courses in Manchester
- knowledge Courses in Sheffield
- knowledge Courses in Leeds
- knowledge Courses in Edinburgh
- knowledge Courses in Leicester
- knowledge Courses in Coventry
- knowledge Courses in Bradford
- knowledge Courses in Cardiff
- knowledge Courses in Belfast
- knowledge Courses in Nottingham
