- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
4854 Courses
This English Exam Preparation course is specifically designed for adult learners who are preparing to take an English language proficiency exam, such as IELTS, TOEFL, or Cambridge exams. Participants will focus on developing the necessary skills and strategies to achieve a successful outcome in their chosen exam. Through targeted practice, exam-specific tasks, and personalised feedback, learners will gain confidence and proficiency in all sections of the exam. Course Duration: 12 weeks (48 sessions) Course Objectives: By the end of this course, participants will: 1. Understand the format and requirements of the chosen English language proficiency exam. 2. Develop effective strategies for each section of the exam. 3. Enhance reading, writing, listening, and speaking skills within the context of the exam. 4. Expand vocabulary and improve language accuracy for exam-specific tasks. 5. Practice time management techniques to complete tasks within the allocated time. Course Outline: Week 1: Introduction to the Exam - Introduction to the chosen English language proficiency exam - Overview of the exam format, sections, and scoring criteria - Assessment of participants' current language proficiency and individual learning goals Week 2: Reading Skills and Strategies - Understanding different question types in the reading section - Developing reading skills for comprehension, skimming, and scanning - Practice exercises and strategies to improve speed and accuracy Week 3: Writing Skills and Strategies - Analyzing the writing tasks and requirements of the exam - Developing skills for organising ideas, structuring paragraphs, and coherence - Practice exercises for different writing tasks (essays, reports, letters, etc.) Week 4: Listening Skills and Strategies - Identifying question types and understanding the listening section format - Developing listening skills for main ideas, details, and inference - Practice exercises and strategies for improving listening accuracy and note-taking Week 5: Speaking Skills and Strategies - Understanding the speaking section requirements and assessment criteria - Developing skills for fluency, coherence, and pronunciation - Practice exercises for different speaking tasks (interviews, presentations, etc.) Week 6: Vocabulary Expansion for Exam Tasks - Expanding vocabulary in specific topics and themes relevant to the exam - Practice exercises to reinforce and use new vocabulary effectively - Techniques for deducing meaning from context and using appropriate vocabulary Week 7: Grammar and Language Accuracy - Reviewing and reinforcing essential grammar rules and structures - Identification and correction of common grammar errors in exam tasks - Exercises and activities to improve language accuracy in writing and speaking Week 8: Time Management and Exam Strategies - Strategies for time management and prioritizing tasks during the exam - Practice exercises to improve speed and efficiency in completing tasks - Tips and techniques for maximizing scores in each section Week 9: Mock Exam: Reading and Writing - Simulating a complete reading and writing section of the exam - Timed practice sessions to replicate exam conditions - Detailed feedback and analysis of strengths and areas for improvement Week 10: Mock Exam: Listening and Speaking - Simulating a complete listening and speaking section of the exam - Timed practice sessions to replicate exam conditions - Detailed feedback and analysis of strengths and areas for improvement Week 11: Exam Strategies Review and Practice - Reviewing and reinforcing exam strategies for each section - Targeted practice exercises to address specific challenges and areas of improvement - Individualised feedback and guidance based on participants' performance Week 12: Final Evaluation and Exam Tips - Comprehensive review of all exam sections and strategies - Final evaluation to assess participants' progress and readiness for the exam - Tips for managing test anxiety and mental preparation for the exam Note: This syllabus is a guideline and can be customised based on the specific exam requirements and the needs, interests, and proficiency levels of the participants. The course may also include additional topics or activities to cater to learners' specific goals or areas of focus.

Growth Mindset Training
By Study Plex
Highlights of the Course Course Type: Online Learning Duration: 1 hours Tutor Support: Tutor support is included Customer Support: 24/7 customer support is available Quality Training: The course is designed by an industry expert Recognised Credential: Recognised and Valuable Certification Completion Certificate: Free Course Completion Certificate Included Instalment: 3 Installment Plan on checkout What you will learn from this course? Gain comprehensive knowledge about growth mindset Understand the core competencies and principles of growth mindset Explore the various areas of growth mindset Know how to apply the skills you acquired from this course in a real-life context Become a confident and expert leader or manager Growth Mindset Training Course Master the skills you need to propel your career forward in growth mindset. This course will equip you with the essential knowledge and skillset that will make you a confident leader or manager and take your career to the next level. This comprehensive growth mindset course is designed to help you surpass your professional goals. The skills and knowledge that you will gain through studying this growth mindset course will help you get one step closer to your professional aspirations and develop your skills for a rewarding career. This comprehensive course will teach you the theory of effective growth mindset practice and equip you with the essential skills, confidence and competence to assist you in the growth mindset industry. You'll gain a solid understanding of the core competencies required to drive a successful career in growth mindset. This course is designed by industry experts, so you'll gain knowledge and skills based on the latest expertise and best practices. This extensive course is designed for leader or manager or for people who are aspiring to specialize in growth mindset. Enroll in this growth mindset course today and take the next step towards your personal and professional goals. Earn industry-recognized credentials to demonstrate your new skills and add extra value to your CV that will help you outshine other candidates. Who is this Course for? This comprehensive growth mindset course is ideal for anyone wishing to boost their career profile or advance their career in this field by gaining a thorough understanding of the subject. Anyone willing to gain extensive knowledge on this growth mindset can also take this course. Whether you are a complete beginner or an aspiring professional, this course will provide you with the necessary skills and professional competence, and open your doors to a wide number of professions within your chosen sector. Entry Requirements This growth mindset course has no academic prerequisites and is open to students from all academic disciplines. You will, however, need a laptop, desktop, tablet, or smartphone, as well as a reliable internet connection. Assessment This growth mindset course assesses learners through multiple-choice questions (MCQs). Upon successful completion of the modules, learners must answer MCQs to complete the assessment procedure. Through the MCQs, it is measured how much a learner could grasp from each section. In the assessment pass mark is 60%. Advance Your Career This growth mindset course will provide you with a fresh opportunity to enter the relevant job market and choose your desired career path. Additionally, you will be able to advance your career, increase your level of competition in your chosen field, and highlight these skills on your resume. Recognised Accreditation This course is accredited by continuing professional development (CPD). CPD UK is globally recognised by employers, professional organisations, and academic institutions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. Course Curriculum Introduction & First Concepts Introduction 00:02:00 Awareness & Planning Is Key 00:06:00 Investing In Yourself Investing In Your Physical Health 00:06:00 Investing In Your Mental Health 00:08:00 Attitude For Success Letting Go Of Negativity 00:09:00 Adopting The Attitude Of A Winner 00:09:00 Build People Up - Not Down 00:02:00 Embracing Positivity 00:04:00 Personal Growth Strategies Being Yourself 00:03:00 Finding Your Place & Purpose 00:03:00 Dedicating Time To What Matters 00:05:00 Final Thoughts & Ideas Final Thoughts & Ideas 00:04:00 Final Assessment Assessment - Growth Mindset Training 00:10:00 Obtain Your Certificate Order Your Certificate of Achievement 00:00:00 Get Your Insurance Now Get Your Insurance Now 00:00:00 Feedback Feedback 00:00:00

Unveil the art of crafting captivating stories for the screen through this comprehensive course. From discovering the essentials of screenwriting to mastering character development, plot intricacies, and proper script structure, this program guides you in creating compelling narratives. Dive into research, genre exploration, and dialogue crafting to refine your storytelling prowess. Learn about script formatting, synopses, and treatments, and delve into troubleshooting techniques. Additionally, explore the intersection of screenwriting with the British film industry, comprehend agreements, and gain insights into the earning potential of UK screenwriters. Learning Outcomes: Develop a foundational understanding of screenwriting and its principles. Cultivate creative motivation and generate ideas for screenplays. Master the research and development process to enhance story authenticity. Explore various genres and their unique storytelling elements. Craft engaging plots and narratives that captivate audiences. Create well-rounded characters and authentic dialogues. Comprehend the structure and formatting conventions of screenplays. Learn to draft synopses, outlines, treatments, and troubleshoot common issues. Why buy this Screenwriting, Scriptwriting and Appraisals? Unlimited access to the course for forever Digital Certificate, Transcript, student ID all included in the price Absolutely no hidden fees Directly receive CPD accredited qualifications after course completion Receive one to one assistance on every weekday from professionals Immediately receive the PDF certificate after passing Receive the original copies of your certificate and transcript on the next working day Easily learn the skills and knowledge from the comfort of your home Certification After studying the course materials of the Screenwriting, Scriptwriting and Appraisals you will be able to take the MCQ test that will assess your knowledge. After successfully passing the test you will be able to claim the pdf certificate for £5.99. Original Hard Copy certificates need to be ordered at an additional cost of £9.60. Who is this course for? This Screenwriting, Scriptwriting and Appraisals course is ideal for Aspiring screenwriters eager to learn the art of scriptwriting. Filmmakers seeking to improve their storytelling skills. Creative individuals interested in exploring screenwriting as a medium. Anyone passionate about contributing to the entertainment industry. Prerequisites This Screenwriting, Scriptwriting and Appraisals was made by professionals and it is compatible with all PC's, Mac's, tablets and smartphones. You will be able to access the course from anywhere at any time as long as you have a good enough internet connection. Career path Screenwriter - Average Earnings: Varies widely; freelance earnings per project. Script Consultant - Average Earnings: £20,000 - £40,000 per year. Film or TV Script Editor - Average Earnings: £25,000 - £50,000 per year. Creative Writing Instructor - Average Earnings: £25,000 - £40,000 per year. Film or TV Director - Average Earnings: £30,000 - £60,000+ per year. Author and Novelist (Transition to Screenwriting) - Earnings Vary by Success. Course Curriculum Screenwriting, Scriptwriting and Appraisals Introduction to Screenwriting 00:11:00 Motivation & Ideas 00:28:00 Research & Development 00:20:00 Genre 00:11:00 Story & Plot 00:28:00 Character & Dialogue 00:50:00 Structure & Format 00:59:00 Synopses, Outlines & Treatments 00:09:00 Drafts 00:17:00 Marketing 00:20:00 Troubleshooting 00:19:00 Glossary 00:11:00 Screenwriting and British Film Industry 00:20:00 Agreements 00:52:00 How Much Do UK Screenwriters Earn? 00:19:00 How Much Do UK Screenwriters Earn? 00:13:00 Mock Exam Mock Exam - Screenwriting, Scriptwriting and Appraisals 00:20:00 Final Exam Final Exam - Screenwriting, Scriptwriting and Appraisals 00:20:00

Professional Certificate in Understanding Sources of Finance and Techniques to Manage Global Risk in London 2024
4.9(261)By Metropolitan School of Business & Management UK
The course offers insights into global investment and financial risks and explores the nature of global sources of funds. The learner will be able to employ tools and analysis techniques to identify potential risks and strategies to mitigate the same. After the successful completion of this lecture, you will be able to understand the following: Understanding Sources of Finance and types of finance. Understanding Global Risk and its types. Understanding Financial Risk and its types. Understanding Strategic Financial Management. Risk Assessment Tools & Techniques. Capital Asset Pricing Model. Cost of Capital. WACC. Understanding Risk Mitigation & Risk Mitigation Techniques. VIDEO - Course Structure and Assessment Guidelines Watch this video to gain further insight. Navigating the MSBM Study Portal Watch this video to gain further insight. Interacting with Lectures/Learning Components Watch this video to gain further insight. Understanding Sources of Finance & Techniques to Manage Global Risk Quiz: Understanding Sources Of Finance & Techniques To Manage Global Risk Put your knowledge to the test with this quiz. Read each question carefully and choose the response that you feel is correct. All MSBM courses are accredited by the relevant partners and awarding bodies. Please refer to MSBM accreditation in about us for more details. There are no strict entry requirements for this course. Work experience will be added advantage to understanding the content of the course. The certificate is designed to enhance the knowledge of the learner in the field. This certificate is for everyone eager to know more and gets updated on current ideas in their respective field. We recommend this certificate for the following audience. CEO, Director, Manager, Supervisor Finance Manager Risk Manager Change Manager Organizational Behaviour Specialist Operations Manager Team Lead Average Completion Time 2 Weeks Accreditation 1 CPD Hour Level Advanced Start Time Anytime 100% Online Study online with ease. Unlimited Access 24/7 unlimited access with pre-recorded lectures. Low Fees Our fees are low and easy to pay online.

Professional Certificate in Understanding Cost System and its Classification in London 2024
4.9(261)By Metropolitan School of Business & Management UK
The course explores the nature and behaviour of cost in an organization and the approaches used to report and manage it. The course aims to equip the learner with an understanding of the essential terminologies and concepts used in costing so that the learner is able to identify costs, interpret the related information and use the costing information in decision making. After the successful completion of this lecture, you will be able to understand the following: The concept of the Costing System. Various types of costing systems. Different classification of cost. Various cost control of systems. Calculation of cumulative cost variance. Various strategies of pricing policy. VIDEO - Course Structure and Assessment Guidelines Watch this video to gain further insight. Navigating the MSBM Study Portal Watch this video to gain further insight. Interacting with Lectures/Learning Components Watch this video to gain further insight. Understanding cost system and its classification Quiz: Understanding Cost System And Its Classification Put your knowledge to the test with this quiz. Read each question carefully and choose the response that you feel is correct. All MSBM courses are accredited by the relevant partners and awarding bodies. Please refer to MSBM accreditation in about us for more details. There are no strict entry requirements for this course. Work experience will be added advantage to understanding the content of the course. The certificate is designed to enhance the knowledge of the learner in the field. This certificate is for everyone eager to know more and gets updated on current ideas in their respective field. We recommend this certificate for the following audience. CEO, Director, Manager, Supervisor Cost Control Manager Finance Manager Operatons Manager Organizational Behaviour Specialist Financial Analyst Team Lead Average Completion Time 2 Weeks Accreditation 1 CPD Hour Level Advanced Start Time Anytime 100% Online Study online with ease. Unlimited Access 24/7 unlimited access with pre-recorded lectures. Low Fees Our fees are low and easy to pay online.

Level 3 Certificate in Event and Hospitality Management
By Compliance Central
Embark on a captivating journey into the vibrant world of Event and Hospitality Management. Picture this: orchestrating grand galas, coordinating seamless conferences, and curating unforgettable experiences, all while mastering the art of hospitality. This isn't just a course; it's your ticket to unlocking boundless opportunities in the Event and Hospitality Management sphere. Immerse yourself in an educational odyssey that delves deep into the intricacies of event planning and hospitality operations. As you navigate through each module, you'll unravel the secrets to crafting impeccable events and fostering unparalleled guest experiences. Enrich your understanding of event logistics, vendor management, and the dynamic landscape of the hospitality industry. Get ready to elevate your expertise and carve your niche in this exhilarating field. Seize the reins of your future and embark on a transformative journey with our Level 3 Certificate in Event and Hospitality Management. Unleash your potential and become a maestro of events and hospitality! Level 3 Certificate in Event and Hospitality Management Learning Outcomes: Master the art of brainstorming and conceptualizing captivating event ideas. Understand the importance of support staff and technical teams in executing flawless events. Develop proficiency in managing vendors and ensuring seamless collaboration. Acquire essential organizational skills to streamline event planning processes. Explore the nuances of hospitality management and its pivotal role in guest satisfaction. Level 3 Certificate in Event and Hospitality Management Module 01: Types of Events Module 02: Brainstorming Module 03: Support Staff Module 04: Technical Staff Module 05: Vendors Module 06: Get Organized Module 07: Post Event Activities Module 08: Introduction to Hospitality Management Module 09: An Overview of Hotel Industry Module 10: Management of Front Office Operations Module 11: Management of Food and Beverage Operations Module 12: Customer Satisfaction Module 13: E-Hospitality and Technology Certificate of Achievement Endorsed Certificate of Achievement from the Quality Licence Scheme Learners will be able to achieve an endorsed certificate after completing the Event and Hospitality Managementcourse as proof of their achievement. You can order the endorsed certificate for Free to be delivered to your home by post. For international students, there is an additional postage charge of £10. Endorsement The Quality Licence Scheme (QLS) has endorsed this event and hospitality management course for its high-quality, non-regulated provision and training programmes. The QLS is a UK-based organisation that sets standards for non-regulated training and learning. This endorsement means that the event and hospitality management course has been reviewed and approved by the QLS and meets the highest quality standards. Who is this course for? Level 3 Certificate in Event and Hospitality Management The Level 3 Certificate in Event and Hospitality Management is designed for individuals seeking to enhance their skills and knowledge in the dynamic field of Event and Hospitality Management, like - Aspiring Event Planners Hospitality Enthusiasts Career Changers seeking new challenges Professionals aiming for career advancement Graduates with a passion for event coordination Requirements Level 3 Certificate in Event and Hospitality Management To enrol in this Level 3 Certificate in Event and Hospitality Management course, all you need is a basic understanding of the English Language and an internet connection. Career path Welcome to a realm of boundless opportunities! Explore diverse career paths after completing this Event and Hospitality Management course. Embrace the journey ahead in Event and Hospitality Management. Event Coordinator Hotel Manager Conference Planner Restaurant Manager Wedding Planner Certificates CPD Accredited PDF Certificate Digital certificate - Included QLS Endorsed Hard Copy Certificate Hard copy certificate - Included CPD Accredited Hard Copy Certificate Hard copy certificate - £9.99 CPD Accredited Hard Copy Certificate Delivery Charge: Inside the UK: Free Outside of the UK: £9.99 each

A System Approach to Good Pharmacovigilance Practice
By Research Quality Association
Course Information In today's evolving landscape, pharmacovigilance (PV) systems face ongoing challenges due to global, national, and company-specific events. This course focuses on developing personnel equipped to navigate these complexities and improve the safe use of medicinal products. We emphasise continuous global thinking, communication, and strategic planning, ensuring adaptability across various levels. The course explores maintaining PV system functionality while adhering to regulatory requirements. Participants will apply European regulatory standards to enhance and sustain PV system effectiveness, contributing to improvement initiatives and ensuring operational integrity. The course covers PV system intricacies, regulatory compliance, and a 'systems approach' for auditors, quality assurance personnel, and PV practitioners. Through presentations and workshops, attendees gain insights into implementing and maintaining an effective PV system. Who should attend? Auditors Pharmacovigilance Quality System Managers Pharmacovigilance scientists The QPPV. Course benefits Throughout the course delegates will explore application of the legal requirements for the PV system and quality system and how to assure these systems. They will demonstrate their ability to contribute to: A systematic investigation of the pharmacovigilance system and its quality system Examination of how the pharmacovigilance system and quality system interact to achieve compliance. The risk-based approach to auditing the PV system and quality system The maintenance of 'inspection readiness' Explore how to investigate the complex PV system Discussions about how to monitor and maintain the PV system and assure compliance. Course Objectives Clarify what has to be done: Explore application of the legal requirements. Explore how to do what has to be done: Adopt a systemic approach to systematically investigate or implement and maintain the PV system and quality system Examine how a compliant PV system and a compliant quality system interact to achieve compliance with regulatory requirements for PV Explore how to investigate the complexity of the PV system. Discus how to identify what is missing or what needs to be improved: Discuss how to monitor and maintain the PV system and assure compliance. This course will assist delegates with: An understanding of key system principles, A practical approach to implementing, maintaining and monitoring the PV system and its quality system A procedure to share expertise to increase efficiency and confidence. This course is structured to encourage delegates to: Discuss and develop ideas - Share knowledge and experiences - Solve specific problems. By the end of the course delegates will be able to: Understand better the pharmacovigilance system, its quality system and how the components interact to achieve the objectives of pharmacovigilance Investigate, and analyse the pharmacovigilance system and to identify what is missing and what needs to be improved. Tutors Tutors will be comprised of (click the photos for biographies): Jana Hyankova Head of PV Department, IVIGEE Services a.s. Programme Please note timings may be subject to alteration. Day 1 08:30 Welcome, registration, course objectives and introduction to work groups Housekeeping notices, meet other delegates, explore how to work in your work group, course objectives. Clarify the definition and objectives of Pharmacovigilance. 09:30 The Regulatory Framework for Pharmacovigilance Identify the relevant regulations and directives. Explore GVP guidance, structure of the modules and standard format of each module. 10:00 The Pharmacovigilance System Exploration of how to organise what has to be done, communications. Exploration of the structures and processes for pharmacovigilance. 10:30 Break 11:00 Workshop 1 and Feedback Exploring an organisational model of the pharmaceutical company- cooperation between PV and other stakeholders. 12:00 The Quality System for pharmacovigilance Exploration of the structures, processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 13:00 Lunch 14:00 Workshop 2 and Feedback The quality system puzzle. Explore the organisation of the PV quality system and how it interacts with the PV system. 14:30 The Quality System for pharmacovigilance Exploration of the structures processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 15:00 Description of PV System 15:30 Break 15:30 Workshop 3 and Feedback The quality system puzzle Explore the organisation of the PV quality system and how it interacts with the PV system. 16:00 The Pharmacovigilance Safety Master File Construction of the Pharmacovigilance System Master File and its purpose. 17:00 Workshop 3 and Feedback Description of PV System. 18:00 End of Day Day 2 08:30 Drug Safety in the Clinical Trial Environment - Part 1 Information flow and responsibilities of the sponsor. 09:30 Workshop 4 and Feedback Drug Safety in the Clinical Trial environment: Information flow and responsibilities of the sponsor. 10:30 Break 11:00 Drug Safety in the Clinical Trial Environment - Part 2 Information flow and responsibilities of the sponsor. 12:00 Lunch 13:00 Workshop 5 and Feedback Drug Safety in the clinical trial environment: Information flow and responsibilities of the sponsor. 13:30 Processing of Safety Data Exploration of safety data processing, verification, validation, follow up, formatting and collation, reporting requirements, quality and data management. 15:00 Break 15:30 EudraVigilance Exploration of how EudraVigilance supports the PV system. 16:15 Signal Detection and Evaluation/Risk Benefit Assessment: Pharmacovigilance Risk Assessment Committee (PRAC): What is a signal? What are the regulatory requirements? How is signal detection and evaluation conducted? Qualitative and quantitative methods of signal detection. Risk benefit assessment. 17:00 Risk Management Plans A cornerstone of Pharmacovigilance safety communications, direct healthcare professional communication 18:00 End of Day Day 3 08:30 The Pharmacovigilance Risk Assessment Committee (PRAC) Exploration of how good practice is achieved. Composition, role and responsibilities. Examples of referrals. 09:15 Development Safety Update Reports (DSURs): Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:30 Break 11:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 12:00 Workshop 6 and Feedback To explore the compilation and submission of the PSUR. 13:00 Lunch 13:30 Role of the QPPV Exploration of the legal responsibilities of the QPPV and the MAH. 14:30 Break 15:00 Workshop 7 and Feedback To explore the challenges faced by the QPPV. 15:30 End of course Extra Information Face-to-Face Course Course material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device< Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD Points 23 Points Development Level Develop

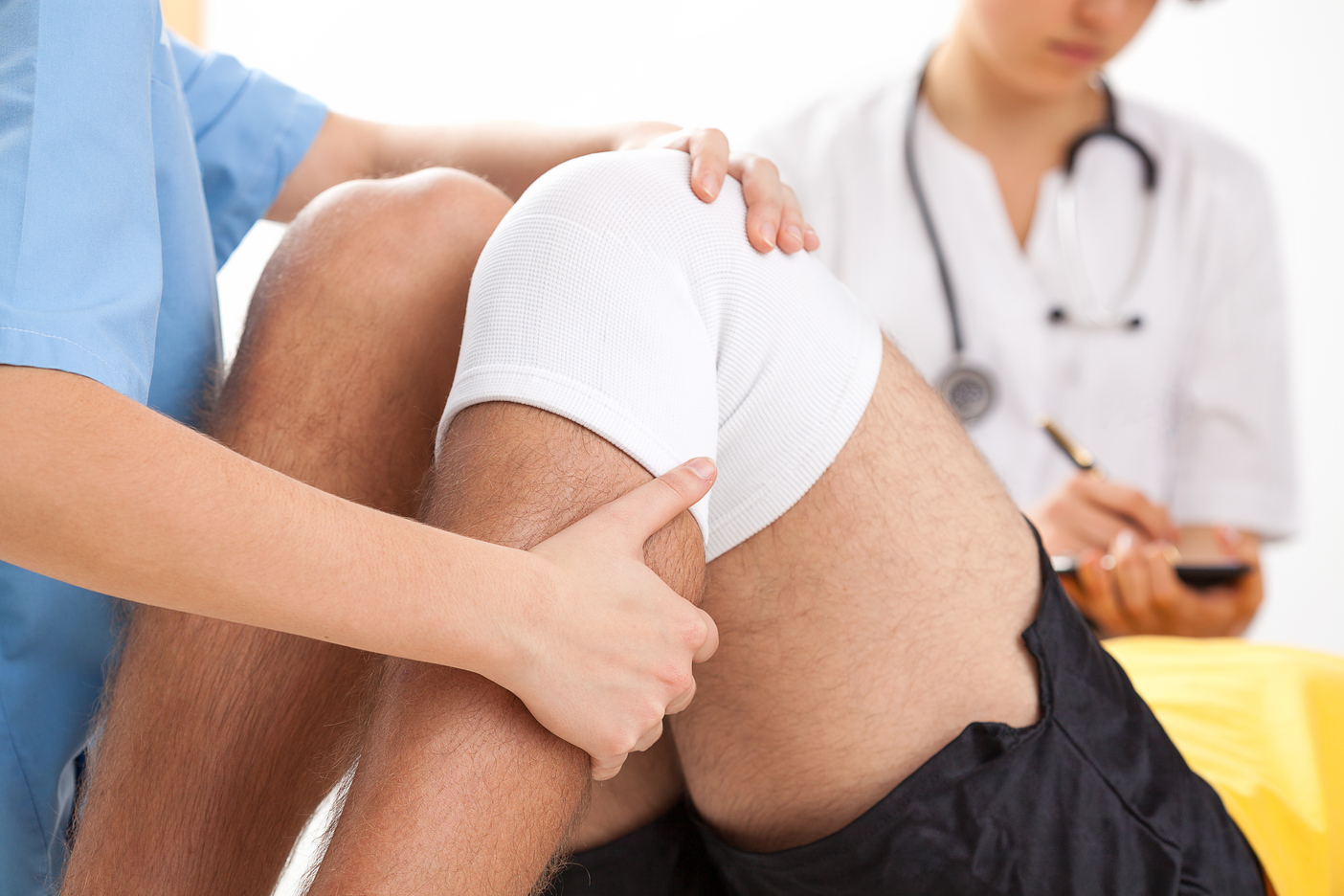
Register on the Joint Health: Maintaining Joints And Reducing Pain today and build the experience, skills and knowledge you need to enhance your professional development and work towards your dream job (Chromologic). Study this course through online learning and take the first steps towards a long-term career.The course consists of a number of easy to digest, in-depth modules, designed to provide you with a detailed, expert level of knowledge. Learn through a mixture of instructional video lessons and online study materials.Receive online tutor support as you study the course, to ensure you are supported every step of the way. Get a digital certificate as a proof of your course completion.The Joint Health: Maintaining Joints And Reducing Pain is incredibly great value and allows you to study at your own pace. Access the course modules from any internet-enabled device, including computers, tablet, and smartphones.The course is designed to increase your employability and equip you with everything you need to be a success. Enrol on the now and start learning instantly!What You Get With The Joint Health: Maintaining Joints And Reducing Pain Receive a e-certificate upon successful completion of the course Get taught by experienced, professional instructors Study at a time and pace that suits your learning style Get instant feedback on assessments 24/7 help and advice via email or live chat Get full tutor support on weekdays (Monday to Friday) Course Design The course is delivered through our online learning platform, accessible through any internet-connected device. There are no formal deadlines or teaching schedules, meaning you are free to study the course at your own pace. You are taught through a combination of Video lessons Online study materials Certification After the successful completion of the final assessment, you will receive a CPD-accredited certificate of achievement. The PDF certificate is for £9.99, and it will be sent to you immediately after through e-mail. You can get the hard copy for £15.99, which will reach your doorsteps by post. Who Is This Course For: The course is ideal for those who already work in this sector or are an aspiring professional. This course is designed to enhance your expertise and boost your CV. Learn key skills and gain a professional qualification to prove your newly-acquired knowledge. Requirements: The online training is open to all students and has no formal entry requirements. To study the Joint Health: Maintaining Joints And Reducing Pain, all your need is a passion for learning, a good understanding of English, numeracy, and IT skills. You must also be over the age of 16. Course Content Section 01: Introduction & First Concepts Introduction and First Concepts 00:01:00 The Importance of Joint Health 00:07:00 Common Joint Problems 00:07:00 Section 02: Exercise, Diet & Weight Exercise Your Joints 00:08:00 Balance Your Diet 00:08:00 Watch Your Weight 00:07:00 Section 03: Best Treatments & Supplements Home Remedies for Joint Pain 00:08:00 Best Joint Supplements 00:10:00 Other Options for Joint Pain 00:08:00 Section 04: Final Thoughts & Ideas Final Thoughts & Ideas 00:01:00 Frequently Asked Questions Are there any prerequisites for taking the course? There are no specific prerequisites for this course, nor are there any formal entry requirements. All you need is an internet connection, a good understanding of English and a passion for learning for this course. Can I access the course at any time, or is there a set schedule? You have the flexibility to access the course at any time that suits your schedule. Our courses are self-paced, allowing you to study at your own pace and convenience. How long will I have access to the course? For this course, you will have access to the course materials for 1 year only. This means you can review the content as often as you like within the year, even after you've completed the course. However, if you buy Lifetime Access for the course, you will be able to access the course for a lifetime. Is there a certificate of completion provided after completing the course? Yes, upon successfully completing the course, you will receive a certificate of completion. This certificate can be a valuable addition to your professional portfolio and can be shared on your various social networks. Can I switch courses or get a refund if I'm not satisfied with the course? We want you to have a positive learning experience. If you're not satisfied with the course, you can request a course transfer or refund within 14 days of the initial purchase. How do I track my progress in the course? Our platform provides tracking tools and progress indicators for each course. You can monitor your progress, completed lessons, and assessments through your learner dashboard for the course. What if I have technical issues or difficulties with the course? If you encounter technical issues or content-related difficulties with the course, our support team is available to assist you. You can reach out to them for prompt resolution.
Register on the Basic Procrastination Solution today and build the experience, skills and knowledge you need to enhance your professional development and work towards your dream job. Study this course through online learning and take the first steps towards a long-term career. The course consists of a number of easy to digest, in-depth modules, designed to provide you with a detailed, expert level of knowledge. Learn through a mixture of instructional video lessons and online study materials. Receive online tutor support as you study the course, to ensure you are supported every step of the way. Get a digital certificate as a proof of your course completion. The Basic Procrastination Solution is incredibly great value and allows you to study at your own pace. Access the course modules from any internet-enabled device, including computers, tablet, and smartphones. The course is designed to increase your employability and equip you with everything you need to be a success. Enrol on the now and start learning instantly! What You Get With The Basic Procrastination Solution Receive a e-certificate upon successful completion of the course Get taught by experienced, professional instructors Study at a time and pace that suits your learning style Get instant feedback on assessments 24/7 help and advice via email or live chat Get full tutor support on weekdays (Monday to Friday) Course Design The course is delivered through our online learning platform, accessible through any internet-connected device. There are no formal deadlines or teaching schedules, meaning you are free to study the course at your own pace. You are taught through a combination of Video lessons Online study materials Certification After the successful completion of the final assessment, you will receive a CPD-accredited certificate of achievement. The PDF certificate is for £9.99, and it will be sent to you immediately after through e-mail. You can get the hard copy for £15.99, which will reach your doorsteps by post. Who Is This Course For: The course is ideal for those who already work in this sector or are an aspiring professional. This course is designed to enhance your expertise and boost your CV. Learn key skills and gain a professional qualification to prove your newly-acquired knowledge. Requirements: The online training is open to all students and has no formal entry requirements. To study the Basic Procrastination Solution, all your need is a passion for learning, a good understanding of English, numeracy, and IT skills. You must also be over the age of 16. Course Content Unit 01: First Concepts and Brain Chemistry Introduction and First Concepts 00:02:00 The Science behind Procrastination 00:09:00 How Procrastination Can Destroy You 00:09:00 Unit 02: How to Beat Beating Procrastination Manage Your Time - Manage Your Life er 00:11:00 How Highly Successful Individual 00:06:00 Unit 03: Additional Strategies for Beating Procrastination Good Vs Bad Procrastination 00:08:00 Don't Be Afraid to Say No 00:06:00 Unit 04: Final Thoughts and Ideas Final Thoughts and ideas 00:02:00 Frequently Asked Questions Are there any prerequisites for taking the course? There are no specific prerequisites for this course, nor are there any formal entry requirements. All you need is an internet connection, a good understanding of English and a passion for learning for this course. Can I access the course at any time, or is there a set schedule? You have the flexibility to access the course at any time that suits your schedule. Our courses are self-paced, allowing you to study at your own pace and convenience. How long will I have access to the course? For this course, you will have access to the course materials for 1 year only. This means you can review the content as often as you like within the year, even after you've completed the course. However, if you buy Lifetime Access for the course, you will be able to access the course for a lifetime. Is there a certificate of completion provided after completing the course? Yes, upon successfully completing the course, you will receive a certificate of completion. This certificate can be a valuable addition to your professional portfolio and can be shared on your various social networks. Can I switch courses or get a refund if I'm not satisfied with the course? We want you to have a positive learning experience. If you're not satisfied with the course, you can request a course transfer or refund within 14 days of the initial purchase. How do I track my progress in the course? Our platform provides tracking tools and progress indicators for each course. You can monitor your progress, completed lessons, and assessments through your learner dashboard for the course. What if I have technical issues or difficulties with the course? If you encounter technical issues or content-related difficulties with the course, our support team is available to assist you. You can reach out to them for prompt resolution.

Register on the Minimalist Lifestyle today and build the experience, skills and knowledge you need to enhance your professional development and work towards your dream job. Study this course through online learning and take the first steps towards a long-term career. The course consists of a number of easy to digest, in-depth modules, designed to provide you with a detailed, expert level of knowledge. Learn through a mixture of instructional video lessons and online study materials. Receive online tutor support as you study the course, to ensure you are supported every step of the way. Get an e-certificate as proof of your course completion. The Minimalist Lifestyle is incredibly great value and allows you to study at your own pace. Access the course modules from any internet-enabled device, including computers, tablet, and smartphones. The course is designed to increase your employability and equip you with everything you need to be a success. Enrol on the now and start learning instantly! What You Get With The Minimalist Lifestyle Receive a e-certificate upon successful completion of the course Get taught by experienced, professional instructors Study at a time and pace that suits your learning style Get instant feedback on assessments 24/7 help and advice via email or live chat Get full tutor support on weekdays (Monday to Friday) Course Design The course is delivered through our online learning platform, accessible through any internet-connected device. There are no formal deadlines or teaching schedules, meaning you are free to study the course at your own pace. You are taught through a combination of Video lessons Online study materials Certification Upon successful completion of the course, you will be able to obtain your course completion e-certificate free of cost. Print copy by post is also available at an additional cost of £9.99 and PDF Certificate at £4.99. Who Is This Course For: The course is ideal for those who already work in this sector or are an aspiring professional. This course is designed to enhance your expertise and boost your CV. Learn key skills and gain a professional qualification to prove your newly-acquired knowledge. Requirements: The online training is open to all students and has no formal entry requirements. To study the Minimalist Lifestyle, all your need is a passion for learning, a good understanding of English, numeracy, and IT skills. You must also be over the age of 16. Course Content Section 01: Introduction & First Concepts Beginning Ideas and Concepts 00:02:00 What Is Minimalist Living 00:06:00 Discover Minimalist Freedom 00:04:00 Section 02: Understanding & Beginning Your Minimalist Lifestyle Benefits of the Minimalist Lifestyle 00:09:00 Personal Benefits of Minimalist Living 00:04:00 How to Start Your Minimalist Lifestyle 00:07:00 Section 03: Minimalism at Work & In Your Personal Life Minimalism at Work 00:03:00 Minimalism in Your Personal Life 00:08:00 Section 04: Living the Minimalist Lifestyle and Dealing with Challenges Balancing Your Minimalist Lifestyle 00:07:00 Conquering Minimalist Challenges 00:07:00 First Steps to a Minimalist Life 00:05:00 Section 05: Conclusion Final Thoughts and Ideas 00:03:00 Frequently Asked Questions Are there any prerequisites for taking the course? There are no specific prerequisites for this course, nor are there any formal entry requirements. All you need is an internet connection, a good understanding of English and a passion for learning for this course. Can I access the course at any time, or is there a set schedule? You have the flexibility to access the course at any time that suits your schedule. Our courses are self-paced, allowing you to study at your own pace and convenience. How long will I have access to the course? For this course, you will have access to the course materials for 1 year only. This means you can review the content as often as you like within the year, even after you've completed the course. However, if you buy Lifetime Access for the course, you will be able to access the course for a lifetime. Is there a certificate of completion provided after completing the course? Yes, upon successfully completing the course, you will receive a certificate of completion. This certificate can be a valuable addition to your professional portfolio and can be shared on your various social networks. Can I switch courses or get a refund if I'm not satisfied with the course? We want you to have a positive learning experience. If you're not satisfied with the course, you can request a course transfer or refund within 14 days of the initial purchase. How do I track my progress in the course? Our platform provides tracking tools and progress indicators for each course. You can monitor your progress, completed lessons, and assessments through your learner dashboard for the course. What if I have technical issues or difficulties with the course? If you encounter technical issues or content-related difficulties with the course, our support team is available to assist you. You can reach out to them for prompt resolution.

Search By Location
- Ideas Courses in London
- Ideas Courses in Birmingham
- Ideas Courses in Glasgow
- Ideas Courses in Liverpool
- Ideas Courses in Bristol
- Ideas Courses in Manchester
- Ideas Courses in Sheffield
- Ideas Courses in Leeds
- Ideas Courses in Edinburgh
- Ideas Courses in Leicester
- Ideas Courses in Coventry
- Ideas Courses in Bradford
- Ideas Courses in Cardiff
- Ideas Courses in Belfast
- Ideas Courses in Nottingham