- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
102 Courses
24 Hour Flash Deal **25-in-1 Lab Analyst & Hematology Advanced Diploma Mega Bundle** Lab Analyst & Hematology Advanced Diploma Enrolment Gifts **FREE PDF Certificate**FREE PDF Transcript ** FREE Exam** FREE Student ID ** Lifetime Access **FREE Enrolment Letter ** Take the initial steps toward a successful long-term career by studying the Lab Analyst & Hematology Advanced Diploma package online with Studyhub through our online learning platform. The Lab Analyst & Hematology Advanced Diploma bundle can help you improve your CV, wow potential employers, and differentiate yourself from the mass. This Lab Analyst & Hematology Advanced Diploma course provides complete 360-degree training on Lab Analyst & Hematology Advanced Diploma. You'll get not one, not two, not three, but twenty-five Lab Analyst & Hematology Advanced Diploma courses included in this course. Plus Studyhub's signature Forever Access is given as always, meaning these Lab Analyst & Hematology Advanced Diploma courses are yours for as long as you want them once you enrol in this course This Lab Analyst & Hematology Advanced Diploma Bundle consists the following career oriented courses: Course 01: Lab Analyst & Basic Hematology Diploma Course 02: Diploma in Laboratory Technician Course 03: Phlebotomy Course 04: Phlebotomist Training Course 05: Pharmacy Assistant & Pharmacy Technician Course 06: Pharmacology Diploma Course 07: Basic Chemistry Course 08: Biochemistry Course 09: Chemical Hygiene and Engineering: Safety in Laboratories Course 10: Health and Safety at Workplace Course 11: Forensic Science Certificate Course 12: Basic Biology Course 13: Medical Terminology Training Course 14: Workplace First Aid Online Training Course Course 15: Principles of Infection Prevention and Control Course 16: Biomedical Science Course 17: Diploma in Water Chemistry Course 18: Clinical Coding Online Course Course 19: Preventing Contamination in the Laboratory: Best Practices in Lab Safety Course 20: Laboratory Safety Course Course 21: Medical Laboratory & Clinical Chemistry Technicians Course 22: Clinical Research Administration: Navigating the Healthcare Landscape Course 23: Healthcare GDPR Certificate Course 24: Comprehensive Training in Sterile Services Management Course 25: Study Skills for Healthcare The Lab Analyst & Hematology Advanced Diploma course has been prepared by focusing largely on Lab Analyst & Hematology Advanced Diploma career readiness. It has been designed by our Lab Analyst & Hematology Advanced Diploma specialists in a manner that you will be likely to find yourself head and shoulders above the others. For better learning, one to one assistance will also be provided if it's required by any learners. The Lab Analyst & Hematology Advanced Diploma Bundle is one of the most prestigious training offered at StudyHub and is highly valued by employers for good reason. This Lab Analyst & Hematology Advanced Diploma bundle course has been created with twenty-five premium courses to provide our learners with the best learning experience possible to increase their understanding of their chosen field. This Lab Analyst & Hematology Advanced Diploma Course, like every one of Study Hub's courses, is meticulously developed and well researched. Every one of the topics is divided into Lab Analyst & Hematology Advanced Diploma Elementary modules, allowing our students to grasp each lesson quickly. The Lab Analyst & Hematology Advanced Diploma course is self-paced and can be taken from the comfort of your home, office, or on the go! With our Student ID card you will get discounts on things like music, food, travel and clothes etc. In this exclusive Lab Analyst & Hematology Advanced Diploma bundle, you really hit the jackpot. Here's what you get: Step by step Lab Analyst & Hematology Advanced Diploma lessons One to one assistance from Lab Analyst & Hematology Advanced Diplomaprofessionals if you need it Innovative exams to test your knowledge after the Lab Analyst & Hematology Advanced Diplomacourse 24/7 customer support should you encounter any hiccups Top-class learning portal Unlimited lifetime access to all twenty-five Lab Analyst & Hematology Advanced Diploma courses Digital Certificate, Transcript and student ID are all included in the price PDF certificate immediately after passing Original copies of your Lab Analyst & Hematology Advanced Diploma certificate and transcript on the next working day Easily learn the Lab Analyst & Hematology Advanced Diploma skills and knowledge you want from the comfort of your home CPD 250 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Lab Analyst & Hematology Advanced Diploma training is suitable for - Students Recent graduates Job Seekers Individuals who are already employed in the relevant sectors and wish to enhance their knowledge and expertise in Lab Analyst & Hematology Advanced Diploma Requirements To participate in this Lab Analyst & Hematology Advanced Diploma course, all you need is - A smart device A secure internet connection And a keen interest in Lab Analyst & Hematology Advanced Diploma Career path You will be able to kickstart your Lab Analyst & Hematology Advanced Diploma career because this course includes various courses as a bonus. This Lab Analyst & Hematology Advanced Diploma is an excellent opportunity for you to learn multiple skills from the convenience of your own home and explore Lab Analyst & Hematology Advanced Diploma career opportunities. Certificates CPD Accredited Certificate Digital certificate - Included CPD Accredited e-Certificate - Free CPD Accredited Hardcopy Certificate - Free Enrolment Letter - Free Student ID Card - Free

24 Hour Flash Deal **25-in-1 Advanced Pharmacy Professional Diploma Mega Bundle** Advanced Pharmacy Professional Diploma Enrolment Gifts **FREE PDF Certificate**FREE PDF Transcript ** FREE Exam** FREE Student ID ** Lifetime Access **FREE Enrolment Letter ** Take the initial steps toward a successful long-term career by studying the Advanced Pharmacy Professional Diploma package online with Studyhub through our online learning platform. The Advanced Pharmacy Professional Diploma bundle can help you improve your CV, wow potential employers, and differentiate yourself from the mass. This Advanced Pharmacy Professional Diploma course provides complete 360-degree training on Advanced Pharmacy Professional Diploma. You'll get not one, not two, not three, but twenty-five Advanced Pharmacy Professional Diploma courses included in this course. Plus Studyhub's signature Forever Access is given as always, meaning these Advanced Pharmacy Professional Diploma courses are yours for as long as you want them once you enrol in this course This Advanced Pharmacy Professional Diploma Bundle consists the following career oriented courses: Course 01: Diploma in Pharmacy Skills Course 02: Pharmacy Assistant & Pharmacy Technician Course 03: Medication Administration: Control and Administration of Medicine Course 04: Medication Administration and Preservation Course 05: Medical Transcription Diploma Course 06: Nurse Prescribing and Medicine Management Course 07: Immunisation Nurse Course 08: Pharmacology Diploma Course 09: Healthcare GDPR Certificate Course 10: Personal Hygiene Course 11: Biomedical Science Course 12: Medical Secretary Certification Course 13: Diploma in Laboratory Technician Course 14: Medical Receptionist Course 15: Medical & Clinical Administrator Course 16: Medical Law Course 17: Essentials of European Medical Device Regulations Course 18: Medical Writing Course 19: Phlebotomy Course 20: Observation Skills for Carers Course 21: Pain Management Course 22: English for Healthcare Course 23: Common Childhood Illnesses & Paediatric First Aid Course 24: Diabetes Awareness Training Course 25: Clinical Research Administration: Navigating the Healthcare Landscape The Advanced Pharmacy Professional Diploma course has been prepared by focusing largely on Advanced Pharmacy Professional Diploma career readiness. It has been designed by our Advanced Pharmacy Professional Diploma specialists in a manner that you will be likely to find yourself head and shoulders above the others. For better learning, one to one assistance will also be provided if it's required by any learners. The Advanced Pharmacy Professional Diploma Bundle is one of the most prestigious training offered at StudyHub and is highly valued by employers for good reason. This Advanced Pharmacy Professional Diploma bundle course has been created with twenty-five premium courses to provide our learners with the best learning experience possible to increase their understanding of their chosen field. This Advanced Pharmacy Professional Diploma Course, like every one of Study Hub's courses, is meticulously developed and well researched. Every one of the topics is divided into Advanced Pharmacy Professional Diploma Elementary modules, allowing our students to grasp each lesson quickly. The Advanced Pharmacy Professional Diploma course is self-paced and can be taken from the comfort of your home, office, or on the go! With our Student ID card you will get discounts on things like music, food, travel and clothes etc. In this exclusive Advanced Pharmacy Professional Diploma bundle, you really hit the jackpot. Here's what you get: Step by step Advanced Pharmacy Professional Diploma lessons One to one assistance from Advanced Pharmacy Professional Diplomaprofessionals if you need it Innovative exams to test your knowledge after the Advanced Pharmacy Professional Diplomacourse 24/7 customer support should you encounter any hiccups Top-class learning portal Unlimited lifetime access to all twenty-five Advanced Pharmacy Professional Diploma courses Digital Certificate, Transcript and student ID are all included in the price PDF certificate immediately after passing Original copies of your Advanced Pharmacy Professional Diploma certificate and transcript on the next working day Easily learn the Advanced Pharmacy Professional Diploma skills and knowledge you want from the comfort of your home CPD 250 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Advanced Pharmacy Professional Diploma training is suitable for - Students Recent graduates Job Seekers Individuals who are already employed in the relevant sectors and wish to enhance their knowledge and expertise in Advanced Pharmacy Professional Diploma Disclaimer: The Pharmacy Technician course is not accredited by the GPhC therefore students completing the course(s) will not be eligible for registration with the GPhC as a pharmacy technician nor will they meet the GPhC's training requirements to work as pharmacy technician. Requirements To participate in this Advanced Pharmacy Professional Diploma course, all you need is - A smart device A secure internet connection And a keen interest in Advanced Pharmacy Professional Diploma Career path You will be able to kickstart your Advanced Pharmacy Professional Diploma career because this course includes various courses as a bonus. This Advanced Pharmacy Professional Diploma is an excellent opportunity for you to learn multiple skills from the convenience of your own home and explore Advanced Pharmacy Professional Diploma career opportunities. Certificates CPD Accredited Certificate Digital certificate - Included CPD Accredited e-Certificate - Free CPD Accredited Hardcopy Certificate - Free Enrolment Letter - Free Student ID Card - Free

24 Hour Flash Deal **33-in-1 Medical Teaching Advanced Diploma Mega Bundle** Medical Teaching Advanced Diploma Enrolment Gifts **FREE PDF Certificate**FREE PDF Transcript ** FREE Exam** FREE Student ID ** Lifetime Access **FREE Enrolment Letter ** Take the initial steps toward a successful long-term career by studying the Medical Teaching Advanced Diploma package online with Studyhub through our online learning platform. The Medical Teaching Advanced Diploma bundle can help you improve your CV, wow potential employers, and differentiate yourself from the mass. This Medical Teaching Advanced Diploma course provides complete 360-degree training on Medical Teaching Advanced Diploma. You'll get not one, not two, not three, but thirty-three Medical Teaching Advanced Diploma courses included in this course. Plus Studyhub's signature Forever Access is given as always, meaning these Medical Teaching Advanced Diploma courses are yours for as long as you want them once you enrol in this course This Medical Teaching Advanced Diploma Bundle consists the following career oriented courses: Course 01: Medical Teaching Course 02: Anatomy and Physiology of Human Body Course 03: Human Anatomy, Physiology and Medical Terminology Diploma Course 04: Biochemistry Course 05: Molecular Genetics Course 06: Neurology, Neurological Assessments & Medication Course 07: Understanding the Immune System Course 08: Radiography Course 09: Dermatology Course 10: Common Childhood Illnesses & Paediatric First Aid Course 11: Paediatric Care Course Course 12: Geriatric Nutrition and Well-being Course 13: Emergency Care Worker Course 14: Paramedicine Study Course 15: Basic Cardiac (Heart) Care Course 16: Medical Jargon for Healthcare Assistant Course 17: Psychological Wellbeing and Crisis Intervention Course 18: Mental Health and Psychiatry Training Course 19: Pharmacology Diploma Course 20: Clinical Pharmacy Certificate Program and Professional Standards Course 21: Medical coding Training Course 22: Medical Transcription Diploma Course 23: Medical & Clinical Administrator Course 24: Nurse Prescribing and Medicine Management Course 25: Infection Control & Medicine Handling Course 26: Medical Law Course 27: Clinical Governance in Adult Care Course 28: Research in Adult Care Course 29: Clinical Research Administration: Navigating the Healthcare Landscape Course 30: Leadership Skills for UK Health Care Professionals Course 31: Health Economics and Health Technology Assessment Course 32: Public Health Course 33: International Healthcare Policy In this exclusive Medical Teaching Advanced Diploma bundle, you really hit the jackpot. Here's what you get: Step by step Medical Teaching Advanced Diploma lessons One to one assistance from Medical Teaching Advanced Diploma professionals if you need it Innovative exams to test your knowledge after the Medical Teaching Advanced Diploma course 24/7 customer support should you encounter any hiccups Top-class learning portal Unlimited lifetime access to all thirty-three Medical Teaching Advanced Diploma courses Digital Certificate, Transcript and student ID are all included in the price PDF certificate immediately after passing Original copies of your Medical Teaching Advanced Diploma certificate and transcript on the next working day Easily learn the Medical Teaching Advanced Diploma skills and knowledge you want from the comfort of your home The Medical Teaching Advanced Diploma course has been prepared by focusing largely on Medical Teaching Advanced Diploma career readiness. It has been designed by our Medical Teaching Advanced Diploma specialists in a manner that you will be likely to find yourself head and shoulders above the others. For better learning, one to one assistance will also be provided if it's required by any learners. The Medical Teaching Advanced Diploma Bundle is one of the most prestigious training offered at StudyHub and is highly valued by employers for good reason. This Medical Teaching Advanced Diploma bundle course has been created with thirty-three premium courses to provide our learners with the best learning experience possible to increase their understanding of their chosen field. This Medical Teaching Advanced Diploma Course, like every one of Study Hub's courses, is meticulously developed and well researched. Every one of the topics is divided into Medical Teaching Advanced Diploma Elementary modules, allowing our students to grasp each lesson quickly. The Medical Teaching Advanced Diploma course is self-paced and can be taken from the comfort of your home, office, or on the go! With our Student ID card you will get discounts on things like music, food, travel and clothes etc. CPD 330 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Medical Teaching Advanced Diploma training is suitable for - Students Recent graduates Job Seekers Individuals who are already employed in the relevant sectors and wish to enhance their knowledge and expertise in Medical Teaching Advanced Diploma Please Note: Studyhub is a Compliance Central approved resale partner for Quality Licence Scheme Endorsed courses. Requirements To participate in this Medical Teaching Advanced Diploma course, all you need is - A smart device A secure internet connection And a keen interest in Medical Teaching Advanced Diploma Career path You will be able to kickstart your Medical Teaching Advanced Diploma career because this course includes various courses as a bonus. This Medical Teaching Advanced Diploma is an excellent opportunity for you to learn multiple skills from the convenience of your own home and explore Medical Teaching Advanced Diploma career opportunities. Certificates CPD Accredited Certificate Digital certificate - Included CPD Accredited e-Certificate - Free CPD Accredited Hardcopy Certificate - Free Enrolment Letter - Free Student ID Card - Free

24 Hour Flash Deal **25-in-1 Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences Mega Bundle** Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences Enrolment Gifts **FREE PDF Certificate**FREE PDF Transcript ** FREE Exam** FREE Student ID ** Lifetime Access **FREE Enrolment Letter ** Take the initial steps toward a successful long-term career by studying the Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences package online with Studyhub through our online learning platform. The Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences bundle can help you improve your CV, wow potential employers, and differentiate yourself from the mass. This Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences course provides complete 360-degree training on Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences. You'll get not one, not two, not three, but twenty-five Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences courses included in this course. Plus Studyhub's signature Forever Access is given as always, meaning these Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences courses are yours for as long as you want them once you enrol in this course This Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences Bundle consists the following career oriented courses: Course 01: Pharmacy Assistant & Pharmacy Technician Course 02: Diploma in Pharmacy Skills Course 03: Medication Administration: Control and Administration of Medicine Course 04: Medication Administration and Preservation Course 05: Medical Transcription Diploma Course 06: Nurse Prescribing and Medicine Management Course 07: Immunisation Nurse Course 08: Pharmacology Diploma Course 09: Healthcare GDPR Certificate Course 10: Biomedical Science Course 11: Medical Secretary Certification Course 12: Diploma in Laboratory Technician Course 13: Medical Receptionist Course 14: Medical & Clinical Administrator Course 15: Medical Law Course 16: Essentials of European Medical Device Regulations Course 17: Medical Writing Course 18: Phlebotomy Course 19: Observation Skills for Carers Course 20: Pain Management Course 21: English for Healthcare Course 22: Common Childhood Illnesses & Paediatric First Aid Course 23: Diabetes Awareness Training Course 24: Clinical Research Administration: Navigating the Healthcare Landscape Course 25: Personal Hygiene The Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences course has been prepared by focusing largely on Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences career readiness. It has been designed by our Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences specialists in a manner that you will be likely to find yourself head and shoulders above the others. For better learning, one to one assistance will also be provided if it's required by any learners. The Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences Bundle is one of the most prestigious training offered at StudyHub and is highly valued by employers for good reason. This Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences bundle course has been created with twenty-five premium courses to provide our learners with the best learning experience possible to increase their understanding of their chosen field. This Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences Course, like every one of Study Hub's courses, is meticulously developed and well researched. Every one of the topics is divided into Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences Elementary modules, allowing our students to grasp each lesson quickly. The Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences course is self-paced and can be taken from the comfort of your home, office, or on the go! With our Student ID card you will get discounts on things like music, food, travel and clothes etc. In this exclusive Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences bundle, you really hit the jackpot. Here's what you get: Step by step Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences lessons One to one assistance from Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciencesprofessionals if you need it Innovative exams to test your knowledge after the Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciencescourse 24/7 customer support should you encounter any hiccups Top-class learning portal Unlimited lifetime access to all twenty-five Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences courses Digital Certificate, Transcript and student ID are all included in the price PDF certificate immediately after passing Original copies of your Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences certificate and transcript on the next working day Easily learn the Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences skills and knowledge you want from the comfort of your home CPD 250 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences training is suitable for - Students Recent graduates Job Seekers Individuals who are already employed in the relevant sectors and wish to enhance their knowledge and expertise in Pharmacy technician Training: Advanced Skills in Pharmaceutical Sciences Requirements To participate in this Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences course, all you need is - A smart device A secure internet connection And a keen interest in Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences Career path You will be able to kickstart your Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences career because this course includes various courses as a bonus. This bundle is an excellent opportunity for you to learn multiple skills from the convenience of your own home and explore Pharmacy Technician Training: Advanced Skills in Pharmaceutical Sciences career opportunities. Certificates CPD Accredited Certificate Digital certificate - Included CPD Accredited e-Certificate - Free CPD Accredited Hardcopy Certificate - Free Enrolment Letter - Free Student ID Card - Free

Junior Clinical Researcher STAR Programme
By European Centre for Clinical Research Training (ECCRT)
How to increase your odds of finding a job in Clinical Research?This STAR Programme will provide the capabilities you need to become a Junior Clinical Researcher. Most employers will require practical experience on the job before considering your application. At ECCRT, we realise that this situation limits the inflow of young, intelligent, and motivated people. To further develop your potential, this STAR Programme combines a series of training and practical internships during a period of one year.

Clinical Research Training for Junior CRAs
By European Centre for Clinical Research Training (ECCRT)
Reasons to attendThis Junior CRA training will train you in the basic, yet crucial areas within clinical monitoring. All typical tasks of a Clinical Research Associate (CRA) will become clear to you, from selecting the investigators until study site close-out, with a great focus on the “monitoring practice” and an introduction on the impact of digitalization. What's included? Documents and materials related to this course are included Globally recognised certificates awarded after test completion This course has been granted PharmaTrain Recognition

Clinical Project Management – Blended
By European Centre for Clinical Research Training (ECCRT)
Reasons to attendHow to ensure a successful clinical trial within timelines and budget? This Clinical Project Management training is a blended course (eLearning + Classroom or Webinars) designed to introduce the ins and outs of managing clinical research projects. The clinical study setting allows you to implement this knowledge immediately within your research projects.What's included? Documents and materials related to this course are included Globally recognised certificates awarded after test completion This course has been granted PharmaTrain Recognition

Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

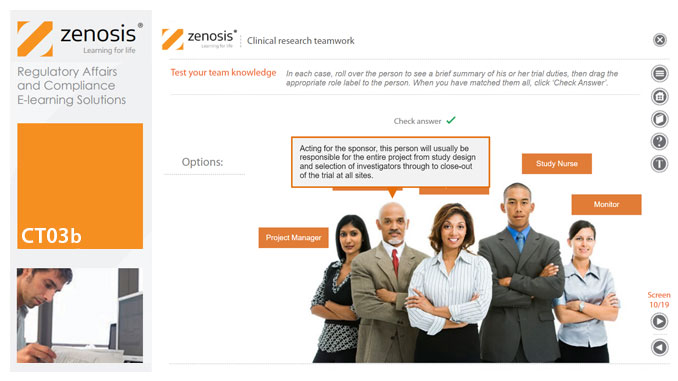
CT03b - Clinical research teamwork
By Zenosis
A clinical trial, particularly a late-phase commercial study, is a major project requiring collaboration between the sponsor and staff or contractor, on the one hand, and the clinical investigator(s) and other healthcare professionals on the other. Good communication among all parties is essential. In this short course we introduce the major roles in a typical clinical research project and outline their duties.
Clinical Research Training for Senior CRAs
By European Centre for Clinical Research Training (ECCRT)
Reasons to attendDo you want to refresh and improve your CRA skills? Do you need some inspiration to boost your clinical trial monitoring techniques and approach? This CRA Training provides for experienced monitors the knowledge and advanced skills to deal with more complex clinical trials and site management issues. This training, supported by eLearning, provides the best outcome allowing you to learn at your own pace. Increase your odds of becoming a senior CRA with this training. What's included? Documents and materials related to this course are included Globally recognised certificates awarded after test completion This course has been granted PharmaTrain Recognition

Search By Location
- Clinical Research Administration Courses in London
- Clinical Research Administration Courses in Birmingham
- Clinical Research Administration Courses in Glasgow
- Clinical Research Administration Courses in Liverpool
- Clinical Research Administration Courses in Bristol
- Clinical Research Administration Courses in Manchester
- Clinical Research Administration Courses in Sheffield
- Clinical Research Administration Courses in Leeds
- Clinical Research Administration Courses in Edinburgh
- Clinical Research Administration Courses in Leicester
- Clinical Research Administration Courses in Coventry
- Clinical Research Administration Courses in Bradford
- Clinical Research Administration Courses in Cardiff
- Clinical Research Administration Courses in Belfast
- Clinical Research Administration Courses in Nottingham