- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
13355 Courses
Duty of Care
By Prima Cura Training
The duty of care is a legal requirement and comes with the job role for any Care worker. It is part of the code of conduct for healthcare support workers and adult social care workers in England and applies as soon as someone receives treatment or care. Employees also have a duty of care to other workers.

BOHS RP404 Refresher - Air Sampling of Asbestos and MMMF and Requirements for a Certificate of Reoccupation Following Clearance of Asbestos
By Airborne Environmental Consultants Ltd
P402 Surveying and sampling strategies for asbestos in buildings is the industry standard qualification for asbestos surveyors. In addition to holding the qualification, asbestos surveyors are required to undertake and provide evidence of annual refresher training. Previously, BOHS provided two Refresher courses for this purpose: P402RM (Management) and P402RRD (Refurbishment and Demolition). This new course, RP402 Refresher - Surveying and Sampling Strategies for Asbestos in Buildings, replaces P402RM and P402RRD. RP402 Refresher enables candidates to revise and update their knowledge on all types of asbestos surveys, and to receive a certificate of course completion by passing a written examination, which covers both the theory and practice of surveying for asbestos in buildings.

2 Day Supervising First Aid for Mental Health
By Prima Cura Training
2 Day Supervising First Aid for Mental Health course is a specialized program catering to leaders and supervisors, equipping them with essential skills to foster a mentally healthy work environment.

The course helps participants understand the role of demand and inventory planning in the wider context of supply chain management. It aims to demonstrate how to improve the alignment between supply and demand to maintain good levels of customer service and on-shelf availability whilst eliminating excess stock and reducing inventory investment. PARTICIPANTS WILL LEARN HOW TO: • Understand the role of demand management and its benefits • Identify the key demand characteristics and patterns; learn how to use them to improve forecast accuracy • Develop an understanding of key qualitative and quantitative forecasting methods • Learn how to conduct fundamental inventory analyses with a view to achieving the appropriate trade-off between stock and service level COURSE TOPICS INCLUDE: The role of Demand Management • The end-to-end view of Supply Chain Management • Demand Characteristics and the Product Life Cycle • Demand patterns • Push and pull systems Background to forecasting • The forecasting Process • Time-series methods of forecasting • Calculating forecast errors Inventory Analysis • Categorisation of stock • ABC Analysis • Economic order quantity and minimum order quantity • Safety stock and stock cover Inventory Management • Materials requirements planning (MRP) • Stock replenishment systems • Practical inventory management • The cost of managing stock

Lead, Empower & Thrive
By Genos International Europe
An instructor-led leadership learning programme based on emotional intelligence and social neuroscience, designed to boost leadership 'PowerSkills.' A practical programme that provides leaders with a learning journey that equips them with the tools and techniques to connect, empathise, communicate effectively, build employee engagement and influence.

Professional Certificate Course in Introduction to Scheduling In Supply Chain Operations in London 2024
4.9(261)By Metropolitan School of Business & Management UK
This Professional Certificate Course in Introduction to Scheduling in Supply Chain Operations covers the fundamentals of operation scheduling, its objectives, functions, and types. It also covers different approaches to scheduling, load work centers, and sequencing methods. The course introduces performance measures and ways to minimize scheduling difficulties. Additionally, the course focuses on the Theory of Constraints, including its principles, metrics, and scheduling multiple resources. Students will learn about time-cost trade-offs and scheduling services. By the end of this course, students will have a comprehensive understanding of scheduling and optimization techniques in the supply chain. This Professional Certificate Course provides a comprehensive understanding of scheduling and optimization techniques in the supply chain operations. The course covers the theory of constraints, load work centers, sequencing methods, and approaches to scheduling. After the successful completion of the course, you will be able to learn about the following, Operation Scheduling, its Objectives, functions, and types. Approaches to scheduling and Load Work Centres, along with concept and method of sequencing, performance measures, and minimizing scheduling difficulties. Theory of Constraints which includes principles, metrics used, Scheduling Services, Scheduling Multiple Resources, and Time-Cost Trade-Offs. This Professional Certificate Course in Introduction to Scheduling in Supply Chain Operations covers the fundamentals of operation scheduling, its objectives, functions, and types. It also covers different approaches to scheduling, load work centers, and sequencing methods. The course introduces performance measures and ways to minimize scheduling difficulties. Additionally, the course focuses on the Theory of Constraints, including its principles, metrics, and scheduling multiple resources. Students will learn about time-cost trade-offs and scheduling services. By the end of this course, students will have a comprehensive understanding of scheduling and optimization techniques in the supply chain. VIDEO - Course Structure and Assessment Guidelines Watch this video to gain further insight. Navigating the MSBM Study Portal Watch this video to gain further insight. Interacting with Lectures/Learning Components Watch this video to gain further insight. Introduction to Scheduling In Supply Chain Operations Self-paced pre-recorded learning content on this topic. Introduction to Scheduling In Supply Chain Operations Put your knowledge to the test with this quiz. Read each question carefully and choose the response that you feel is correct. All MSBM courses are accredited by the relevant partners and awarding bodies. Please refer to MSBM accreditation in about us for more details. There are no strict entry requirements for this course. Work experience will be added advantage to understanding the content of the course. The certificate is designed to enhance the learner's knowledge in the field. This certificate is for everyone eager to know more and get updated on current ideas in their respective field. We recommend this certificate for the following audience Supply chain professionals Operations managers Logistics professionals Production managers Inventory managers Procurement managers Anyone interested in supply chain scheduling and optimization Average Completion Time 2 Weeks Accreditation 3 CPD Hours Level Advanced Start Time Anytime 100% Online Study online with ease. Unlimited Access 24/7 unlimited access with pre-recorded lectures. Low Fees Our fees are low and easy to pay online.

Qualified and experienced Brazilian Portuguese teacher - online or face-to-face lessons.
By Eddie Santos
I am a qualified and experienced Brazilian Portuguese teacher with over 30 years of experience teaching children, teenagers and adults. I teach Brazilian Portuguese for general purposes but also prepare students for GCSE, A-levels and CELPE-Bras. I have also been an exame conductor for many years in several schools across London. I love teaching and helping my students speaking as a native Brazilian.

CT04c - Clinical trial preparation
By Zenosis
The demands on quality from clinical trials are increasing. Quantitative aspects of clinical trials, such as the mass of study data to be collected, the multiple investigational sites, and the need to meet predetermined timelines, often supersede qualitative features. Therefore, addressing basic requirements for quality management is essential when preparing a clinical trial. This short course describes the core elements required for the establishment of a clinical trial and provides an overview of the role of the sponsor in supporting and improving trial quality.
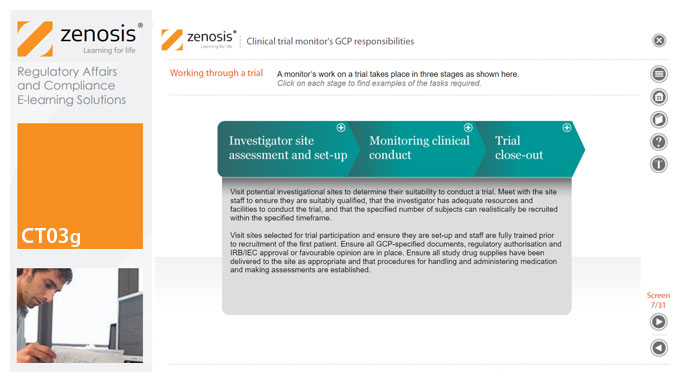
CT03g - Clinical trial monitor’s GCP responsibilities
By Zenosis
A clinical trial monitor acts on behalf of the sponsor to support investigational site personnel, verify the accuracy of data recorded, and ensure that the trial is conducted in compliance with the protocol, GCP and other study specific requirements. He or she acts as the ‘eyes and ears’ of the sponsor at the investigational site and provides the main channel of communication between sponsor and investigator. This short course explores the responsibilities of the monitor and provides insight into key challenges. We discuss assessment of investigators and investigational sites, education and trial initiation, monitoring of clinical conduct, including CRF review and source document verification, and trial close-out. We discuss noncompliance and how to deal with it.
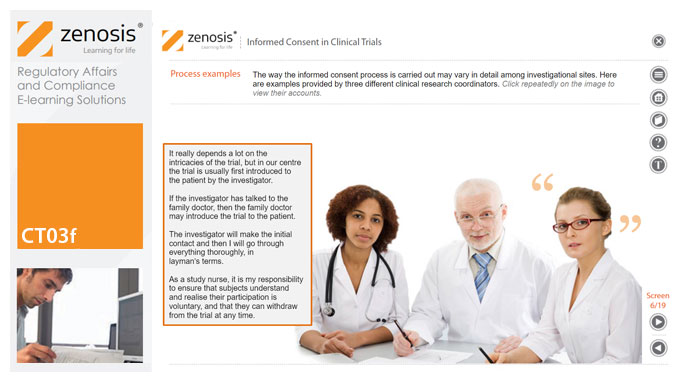
CT03f - Informed consent in clinical trials
By Zenosis
Informed consent in clinical research is an ethical and regulatory requirement. A research subject must enter a study voluntarily, be informed about risks and benefits, and understand the difference between investigation and treatment. Subjects must not be coerced into enrolment, nor must they be enticed by exaggerated claims of benefit. Before they can enrol, all potential subjects must agree, in writing, to participate. In addition to ethical and regulatory imperatives, the potential for litigation by subjects further highlights the importance of rigorous adherence to informed consent principles. In this short course we set out the principles and requirements and provide examples of practical issues confronting healthcare professionals and subjects.
Search By Location
- Safety Courses in London
- Safety Courses in Birmingham
- Safety Courses in Glasgow
- Safety Courses in Liverpool
- Safety Courses in Bristol
- Safety Courses in Manchester
- Safety Courses in Sheffield
- Safety Courses in Leeds
- Safety Courses in Edinburgh
- Safety Courses in Leicester
- Safety Courses in Coventry
- Safety Courses in Bradford
- Safety Courses in Cardiff
- Safety Courses in Belfast
- Safety Courses in Nottingham