- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
8017 Courses
Overview: ***Limited Time Offer*** ★★★ Enrolment Gift: Get Hard Copy + PDF Certificates + Transcript + Student ID Card worth £180 - Enrol Now! ★★★ Gain the skills and abilities of a Phlebotomy to understand the role and responsibilities pertaining to a phlebotomist. Learn about the challenges that come with this role, understand laboratory requisitions, how to prevent infections, perform a dermal puncture, and learn about bleeding time and platelet functions and risk management. This Phlebotomy course is comprehensive and will provide an overview of phlebotomy and then move on to the specifics of a range of areas that you need to be thorough and confident in when carrying out the tasks and duties of a phlebotomist. Along with this Phlebotomy bundle, you will get 10 premium courses, an original Hardcopy, 11 PDF Certificates (Main Course + Additional Courses) Student ID card as gifts. Courses included with this Phlebotomy Training Bundle: Course 01: Phlebotomy Diploma Course 02: Medication Administration Level 4 Course 03: Sterile Services Technician Training Level 4 Course 04: Infection Prevention Training Course 05: Community Practice Nurse Course 06: Nurse Prescribing Diploma Course 07: Human Anatomy and Physiology Course 08: Adult Nursing Diploma Course 09: First Aid Training Course 10: Personal Hygiene Course 11: Health Awareness and Social Care Training Key Features of the Phlebotomy Training Course: FREE Phlebotomy Training CPD-accredited certificate Get a free student ID card with Phlebotomy Training training (£10 applicable for international delivery) Lifetime access to the Phlebotomy Training course materials The Phlebotomy Training program comes with 24/7 tutor support Get instant access to this Phlebotomy Training course Learn Phlebotomy Training training from anywhere in the world The Phlebotomy Training training is affordable and simple to understand The Phlebotomy Training training is an entirely online So enrol now in this Phlebotomy Training Bundle to advance your career! Description: This Phlebotomy Training diploma offers learners the opportunity to acquire the skills that are highly valued in this field. With this Certification, graduates are better positioned to pursue career advancement and higher responsibilities within the Phlebotomy setting. The skills and knowledge gained from this Phlebotomy Training course will enable learners to make meaningful contributions to related fields, impacting their experiences and long-term development. ★★★ Course Curriculum of the Phlebotomy Training Bundle ★★★ Course 01: Phlebotomy Diploma Module 01: Introduction to Phlebotomy Module 02: Blood Circulation, Function, and Composition Module 03: Phlebotomy Equipment Module 04: Routine Venipuncture Module 05: Venipuncture Complications and Pre-Examination Variables Module 06: Dermal Puncture Module 07: Quality Assessment and Management in Phlebotomy Module 08: Special Blood Collection Procedure Module 09: Infection Control and Risk Management =========>>>>> And 10 More Courses <<<<<========= How will I get my Phlebotomy Training Certificate? After successfully completing the Phlebotomy course you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (Previously it was £9.99*11 = £109) Hard Copy Certificate: Free (For The Title Course: Previously it was £14.99) So, enrol now in this Phlebotomy Training Bundle to advance your career! Who is this course for? This course is regularly updated to align with industry standards and practices, ensuring you receive the most relevant and up-to-date knowledge. Don't just take our word for it. This Phlebotomy Training course has received top reviews from our learners, who have praised its comprehensive content and ability to provide a solid foundation for a successful career in phlebotomy. Requirements No prior experience or specific qualifications are required to enrol in this Phlebotomy Training course. Career Path Phlebotomy Training - CPD Certified This Phlebotomy course will improve your eligibility for a number of jobs across the healthcare and medical industries such as: Phlebotomist: £22,000 - £26,000 per year Medical Laboratory Assistant: £16,000 - £20,000 per year Blood Donor Technician: £18,000 - £22,000 per year Clinical Research Assistant: £20,000 - £24,000 per year Healthcare Assistant: £17,000 - £22,000 per year Laboratory Technician: £18,000 - £24,000 per year Pathology Collector: £18,000 - £22,000 per year Certificates Digital certificate - Included Hard copy certificate - Included You will get the Hard Copy certificate for the title course (Phlebotomy Training) absolutely Free! Other Hard Copy certificates are available for £14.99 each. Please Note: The delivery charge inside the UK is £3.99, and the international students must pay a £9.99 shipping cost.

Photography Advanced Diploma : Digital, Fashion, Product, Outdoor & Street Photography
4.5(3)By Studyhub UK
In today's dynamic visual landscape, photography stands as both an art form and a crucial means of communication. As the demand for captivating images continues to soar, there's a growing need for skilled photographers who can navigate diverse genres. In the UK, where visual storytelling is at its peak, mastering the intricacies of digital, fashion, product, outdoor, and street photography is paramount. Our comprehensive CPD Certified course caters to this demand, offering an immersive journey through the lens, from the basics of digital photography to advanced techniques like aerial imaging and astrophotography. This Photography: Digital, Fashion, Product, Outdoor & Street Photography - CPD Certified Bundle Consists of the following Premium courses: Course 01: Photography : Digital Photography Course 02: Portrait Photography Masterclass Course 03: Outdoor Photography Course 04: Flash Photography: Off-Camera Flash Course 05: Product Photography Course 06: Creating 360º Photos with Photoshop Course 07: Smartphone Photography Online Course Course 08: Event Management & Wedding Photography Course 09: Night Photography Course Course 10: Family Portrait Photography Masterclass Course 11: Stage Lighting Course 12: Basic Food Photography Course 13: Drone Photography: Aerial Imaging and Cinematography Course 14: Fashion Photography: Capturing Style and Elegance Course 15: Adobe Lightroom CC Course 16: Mastering the Art and Science of Astrophotography Course 17: Professional After Effect for Photography Course 18: Adobe Photoshop CC Course 19: Digital Art - Sketching In Photoshop for Beginners Course 20: Digital Painting Course 10 Extraordinary Career Oriented courses that will assist you in reimagining your thriving techniques- Course 01: Effective Communication Skills Diploma Course 02: Business Networking Skills Course 03: Influencing and Negotiation Skills Course 04: Delegation Skills Training Course 05: Time Management Course 06: Leadership Skills Training Course 07: Decision Making and Critical Thinking Online Course Course 08: Emotional Intelligence and Social Management Diploma Course 09: Assertiveness Skills Course 10: Touch Typing Complete Training Diploma Learning Outcomes: Upon completion of this Photography: Digital, Fashion, Product, Outdoor & Street Photography - CPD Certified bundle, you should be able to: Capture stunning portraits with advanced techniques and styles. Excel in outdoor photography, leveraging natural light and landscapes. Master off-camera flash for impactful and dynamic images. Showcase products with finesse through expert product photography. Navigate the realms of smartphone and night photography adeptly. Elevate your skills in astrophotography and aerial imaging. This course is designed to empower aspiring photographers with a versatile skill set, addressing contemporary challenges and trends. Unveil the secrets of outdoor photography, conquer the intricacies of flash photography, and delve into the realms of product and fashion photography. From mastering Adobe Lightroom and Photoshop to the intricacies of 360º and drone photography, this course equips you with the tools to transform your vision into captivating imagery. Join us on a transformative odyssey that transcends conventional photography boundaries, embracing both the timeless and the cutting-edge. CPD 300 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Enthusiasts seeking to master diverse photography genres. Content creators aiming to enhance their visual storytelling. Individuals keen on exploring the creative potential of photography. Professionals desiring to broaden their skill set in imaging. Hobbyists aspiring to turn their passion into a refined craft. Please Note: Studyhub is a Compliance Central approved resale partner for Quality Licence Scheme Endorsed courses. Requirements To participate in this Photography course, all you need is - A smart device A secure internet connection And a keen interest in Photography Career path Portrait Photographer: •25,000 - •35,000 Product Photographer: •28,000 - •40,000 Wedding Photographer: •22,000 - •30,000 Fashion Photographer: •28,000 - •45,000 Event Photographer: •20,000 - •28,000 Aerial Photographer: •30,000 - •45,000 Certificates CPD Accredited Certificate Digital certificate - Included CPD Accredited e-Certificate - Free CPD Accredited Hardcopy Certificate - Free Enrolment Letter - Free Student ID Card - Free

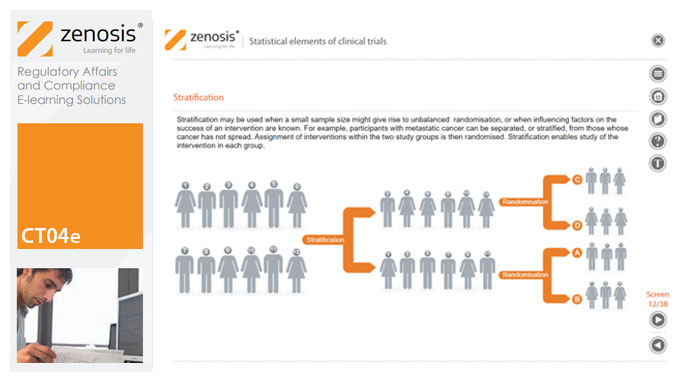
CT04e - Statistical elements of clinical trials
By Zenosis
Analytical statistical elements are essential concepts in the design of clinical trials. This analysis helps us to understand whether a conclusion from a study of a sample of the target population applies generally to that population as a whole. In particular, it helps us to answer the question: Did the treatment effect in the given study occur just by chance? The statistical elements of a well-controlled study minimise the chances of drawing the wrong conclusions, by providing clear thresholds for such errors. The basic statistical elements of a clinical trial include eligibility criteria, randomisation, sample size, power, and blinding, and these are discussed in this short course.
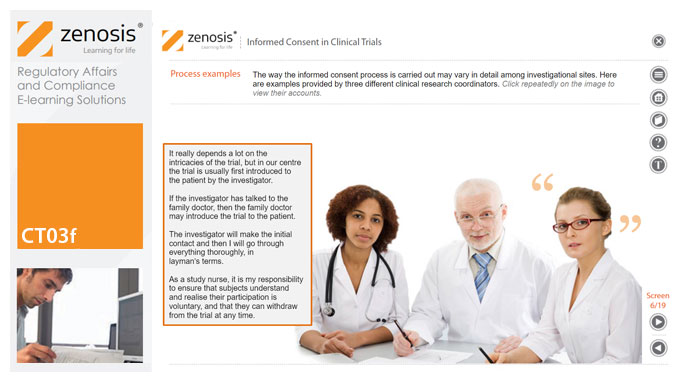
CT03f - Informed consent in clinical trials
By Zenosis
Informed consent in clinical research is an ethical and regulatory requirement. A research subject must enter a study voluntarily, be informed about risks and benefits, and understand the difference between investigation and treatment. Subjects must not be coerced into enrolment, nor must they be enticed by exaggerated claims of benefit. Before they can enrol, all potential subjects must agree, in writing, to participate. In addition to ethical and regulatory imperatives, the potential for litigation by subjects further highlights the importance of rigorous adherence to informed consent principles. In this short course we set out the principles and requirements and provide examples of practical issues confronting healthcare professionals and subjects.
CT03d - Clinical trial sponsor’s GCP responsibilities
By Zenosis
The sponsor of a clinical trial takes responsibility for its initiation, management, and/or financing. A sponsor may transfer any or all of the sponsor’s trial-related duties and functions to a contract research organisation, but the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor. Duties and functions discussed in this short course include trial design, selection of investigators, QA and QC, data handling and record keeping, finance and compensation, regulatory submissions, management of investigational product(s), safety reporting, monitoring, audit, dealing with noncompliance, and clinical trial reports. ICH guideline E6 (revision 2) encourages sponsors to adopt a risk-based approach to managing the quality of trials. We discuss this approach in general, and aspects such as risk-based monitoring in particular.
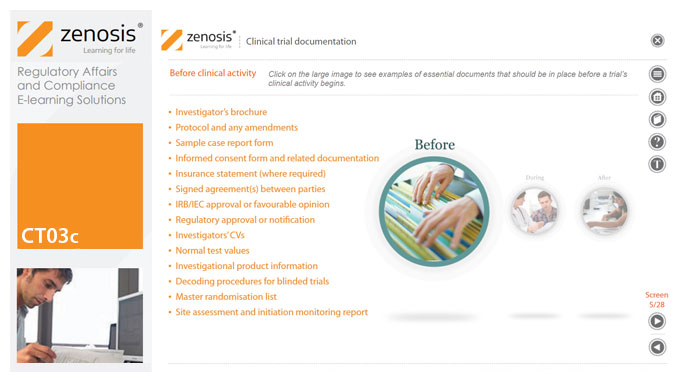
CT03c - Clinical trial documentation
By Zenosis
Regulatory authorities tend to abide by the maxim that ‘If it isn’t documented, it didn’t happen’. Rigorous documentation of all aspects of a clinical trial is necessary to provide evidence of GCP and compliance with regulatory requirements, as well as enabling effective management of the trial. In this short course we describe important examples of the documents designated by ICH GCP as essential to the conduct of a clinical trial.
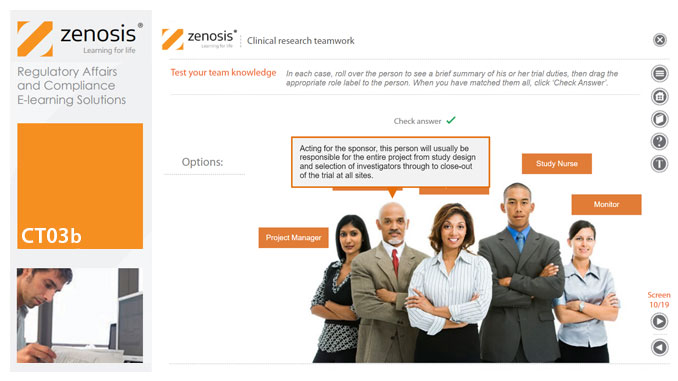
CT03b - Clinical research teamwork
By Zenosis
A clinical trial, particularly a late-phase commercial study, is a major project requiring collaboration between the sponsor and staff or contractor, on the one hand, and the clinical investigator(s) and other healthcare professionals on the other. Good communication among all parties is essential. In this short course we introduce the major roles in a typical clinical research project and outline their duties.
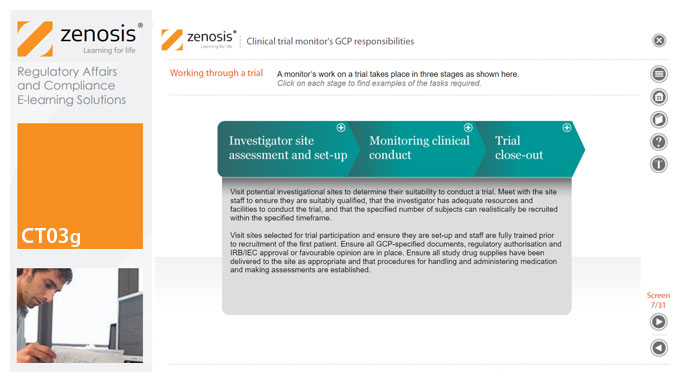
CT03g - Clinical trial monitor’s GCP responsibilities
By Zenosis
A clinical trial monitor acts on behalf of the sponsor to support investigational site personnel, verify the accuracy of data recorded, and ensure that the trial is conducted in compliance with the protocol, GCP and other study specific requirements. He or she acts as the ‘eyes and ears’ of the sponsor at the investigational site and provides the main channel of communication between sponsor and investigator. This short course explores the responsibilities of the monitor and provides insight into key challenges. We discuss assessment of investigators and investigational sites, education and trial initiation, monitoring of clinical conduct, including CRF review and source document verification, and trial close-out. We discuss noncompliance and how to deal with it.
CT04a - Clinical trials in drug development
By Zenosis
New drug development requires major investment in capital, human resources and technical expertise. Strict adherence to regulations on testing and manufacturing standards is also required before a new drug can be marketed. One of the greatest challenges in conducting clinical trials is that of efficiency. As trials become more comprehensive, involving large numbers of participants globally, their duration is prolonged and costs increase. The longer trials last, the shorter is the patent life remaining after market approval and the longer patients must wait for the new product. This short course covers the key components of clinical trials and how these requirements interact with the drug development cycle.

CT03a - ICH, harmonisation, and principles of Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data in applications for marketing approval. The International Council for Harmonisation's guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice. In this short course we describe the ICH’s role in the harmonisation of regulations, introduce its guideline E6, and set out the principles of GCP.
Search By Location
- Medicine and Nursing Courses in London
- Medicine and Nursing Courses in Birmingham
- Medicine and Nursing Courses in Glasgow
- Medicine and Nursing Courses in Liverpool
- Medicine and Nursing Courses in Bristol
- Medicine and Nursing Courses in Manchester
- Medicine and Nursing Courses in Sheffield
- Medicine and Nursing Courses in Leeds
- Medicine and Nursing Courses in Edinburgh
- Medicine and Nursing Courses in Leicester
- Medicine and Nursing Courses in Coventry
- Medicine and Nursing Courses in Bradford
- Medicine and Nursing Courses in Cardiff
- Medicine and Nursing Courses in Belfast
- Medicine and Nursing Courses in Nottingham