- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
304 Courses
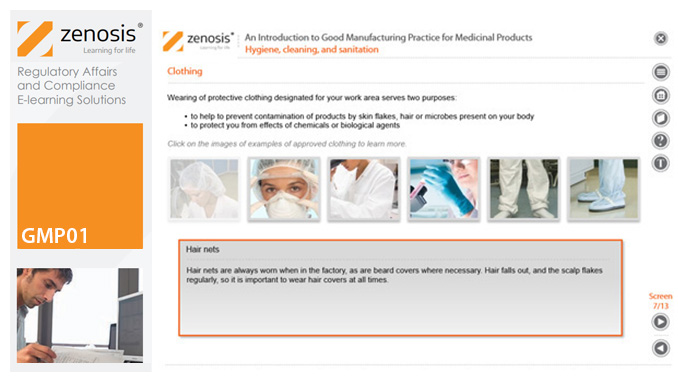
GMP01: An Introduction to Good Manufacturing Practice for Medicinal Products
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. The rules may be written into law or set out in guidance documents from regulatory authorities. Regulators will not allow medicinal products to be placed, or to remain, on the market in their country unless the products can be shown to be manufactured in compliance with GMP. To this end, they carry out inspections of manufacturing plants. Companies that persistently commit serious breaches of GMP requirements have suffered huge fines.
Join our Pharmacy Assistant and Technician Diploma course and start your exciting career journey in pharmacy. You will learn how to counsel patients, handle prescriptions, and manage inventory. This course is ideal for anyone who wants to become a pharmacy professional, a healthcare practitioner, or a pharmaceutical service provider. You will get expert guidance, CPD certification, and flexible study options. Don't miss this opportunity and enroll today!

Eazzy Slim Hypnotherapy, Hypno-No-Jab and Hypnotic Gastic Band.
5.0(26)By The Northern College Of Clinical Hypnotherapy
🌟 Hypnotherapists’ Workshop Day: The Eazzy Slim System & The Hypnotic Gastric Band 🌟 Date: 21st of June | Location: Online (Zoom) | Time: 10 am till 4 pm Are you ready to expand your weight-loss toolkit with powerful, non-invasive techniques that create real and lasting change? Join us for an immersive one-day workshop where we’ll explore the full potential of the Eazzy Slim Programme — a structured, proven system designed specifically for hypnotherapists to help clients achieve sustainable weight loss without the need for medication or surgery. 🔍 What You'll Learn: The Hypno-No-Jab Approach: Discover how to offer clients a highly effective hypnotic alternative to pharmaceutical weight-loss injections like Mounjaro or Ozempic especially important given rising concerns over side effects, long-term use, and the controversial decision to make these drugs available to children. Hypnotic Gastric Band: Learn how to safely and ethically guide clients through a hypnotic gastric band process, mimicking the physical sensation and psychological impact of surgery without any of the risks. The Psychology of Sustainable Change: Explore how to leverage hypnotherapy to reshape beliefs, habits, and identity around eating, self-worth, and health. 🧠 Why It Matters: While medications like Mounjaro can offer temporary results, they often come with side effects, dependency risks, and rebound weight gain once stopped. Likewise, gastric band surgery, once a popular bariatric option, has seen a sharp decline due to its high rate of complications, reoperations, and limited long-term success. Our clients are looking for something different. Something safe. Natural. Sustainable. Empowering. That’s where you come in. 💼 Leave with a Complete Toolkit: Whether you’re working in-person, online, or offering remote packages, this workshop will give you everything you need to start delivering Eazzy Slim straight away, including: Session structure & protocols Client scripts and resources Marketing guidance for positioning your service as a credible alternative to jabs and surgery Q&A and practitioner support 👥 Who This Is For: Qualified hypnotherapists looking to specialise in weight management Practitioners concerned about the long-term health impact of pharmacological weight loss Therapists passionate about offering clients empowering mind-body solutions 🎟️ Secure your place today and become part of the solution. Places are limited to ensure an interactive, high-value experience.
Pharmacy Technician and Pharmaceutical Terminology
By Compliance Central
Are you looking to enhance your Pharmacy Technician and Pharmaceutical Terminology skills? If yes, then you have come to the right place. Our comprehensive course on Pharmacy Technician and Pharmaceutical Terminology will assist you in producing the best possible outcome by mastering the Pharmacy Technician and Pharmaceutical Terminology skills. The Pharmacy Technician and Pharmaceutical Terminology is for those who want to be successful. In the Pharmacy Technician and Pharmaceutical Terminology, you will learn the essential knowledge needed to become well versed in Pharmacy Technician and Pharmaceutical Terminology. Our Pharmacy Technician and Pharmaceutical Terminology starts with the basics of Pharmacy Technician and Pharmaceutical Terminology and gradually progresses towards advanced topics. Therefore, each lesson of this Pharmacy Technician and Pharmaceutical Terminology is intuitive and easy to understand. Why would you choose the Pharmacy Technician and Pharmaceutical Terminology from Compliance Central: Lifetime access to Pharmacy Technician and Pharmaceutical Terminology materials Full tutor support is available from Monday to Friday with the Pharmacy Technician and Pharmaceutical Terminology Learn Pharmacy Technician and Pharmaceutical Terminology skills at your own pace from the comfort of your home Gain a complete understanding of Pharmacy Technician and Pharmaceutical Terminology Accessible, informative Pharmacy Technician and Pharmaceutical Terminology learning modules designed by expert instructors Get 24/7 help or advice from our email and live chat teams with the Pharmacy Technician and Pharmaceutical Terminology bundle Study Pharmacy Technician and Pharmaceutical Terminology in your own time through your computer, tablet or mobile device. A 100% learning satisfaction guarantee with your Pharmacy Technician and Pharmaceutical Terminology Improve your chance of gaining in demand skills and better earning potential by completing the Pharmacy Technician and Pharmaceutical Terminology Pharmacy Technician and Pharmaceutical Terminology Curriculum Breakdown: Module 1: Introduction to Pharmacy Assistant and Pharmacy Technician Module 2: Job Role of Pharmacy Technicians Module 3: Pharmacy Assistant Patient Counselling Guide Module 4: Communication in Pharmacy Settings Module 5: The Pharmacy Team and Practices Module 6: Prescription and Dispensing in Pharmacies Module 7: Dispensing Methods, EPS, Minimising Dispensing Errors in Pharmacies Module 8: Inventory Control and Management in Pharmacies Module 9: Standard Operating Procedures (SOPs) Module 10: Health and Safety Risks Assessment and Pharmaceutical Terminology CPD 10 CPD hours / points Accredited by CPD Quality Standards Who is this course for? The Pharmacy Technician and Pharmaceutical Terminology helps aspiring professionals who want to obtain the knowledge and familiarise themselves with the skillsets to pursue a career in Pharmacy Technician and Pharmaceutical Terminology. It is also great for professionals who are already working in Pharmacy Technician and Pharmaceutical Terminology and want to get promoted at work. Requirements To enrol in this Pharmacy Technician and Pharmaceutical Terminology, all you need is a basic understanding of the English Language and an internet connection. Career path The Pharmacy Technician and Pharmaceutical Terminology will enhance your knowledge and improve your confidence in exploring opportunities in various sectors related to Pharmacy Technician and Pharmaceutical Terminology. Certificates CPD Accredited PDF Certificate Digital certificate - Included CPD Accredited PDF Certificate CPD Accredited Hard Copy Certificate Hard copy certificate - £10.79 CPD Accredited Hard Copy Certificate Delivery Charge: Inside the UK: Free Outside of the UK: £9.99 each

Get Hard Copy + PDF Certificates + Transcript + Student ID Card as a Gift - Enrol Medical Writing, Clinical Coding & Medical Transcription Now Medical writing is the practice of writing scientific documents by writers in the field of medicine. It is one of the fastest developing career opportunities that involve writing topics related to the healthcare and drug development Industry. There is a large demand for Medical Writing, Clinical Coding & Medical Transcription professionals. You get exposed to multiple career opportunities with various contract research organisations, healthcare websites, journals, magazines, pharmaceutical companies, and more. Furthermore, to help you showcase your expertise in Medical Writing, we have prepared a special gift of 1 hardcopy certificate and 1 PDF certificate for the title course completely free of cost. These certificates will enhance your credibility and encourage possible employers to pick you over the rest. This Medical Writing, Clinical Coding & Medical Transcription Bundle Consists of the following Premium courses: Course 01: Medical Writing Course 02: Clinical Coding Course 03: Medical Transcription Course 04: Clinical Data Analysis with SAS Course 05: Medical Terminology Training Course 06: Biomedical Science Course 07: European Medical Device Regulations Course 08: Public Health Course 09: Medical Law Course 10: GDPR in Healthcare Course 11: Content Management Course 12: Write and Publish a Research Paper: Complete Guide v6 Course 13: Improve English Spelling, Punctuation, Grammar and Pronunciation Course 14: Decision Making and Critical Thinking Key features of this Medical Writing, Clinical Coding & Medical Transcription course: This Medical Writing, Clinical Coding & Medical Transcription is CPD QS Accredited Learn from anywhere in the world Lifetime access Medical Writing, Clinical Coding & Medical Transcription is entirely online 24/7 Learner support Enrol now in Medical Writing, Medical Coding & Medical Transcription to advance your career, and use the premium study materials from Apex Learning. How will I get my Medical Writing, Clinical Coding & Medical Transcription Certificate? After successfully completing the Medical Writing, Clinical Coding & Medical Transcription course you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (For The Title Course) Hard Copy Certificate: Free (For The Title Course) CPD 140 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone from any background can enrol in this Medical Writing, Clinical Coding & Medical Transcription Bundle. Requirements This Medical Writing, Clinical Coding & Medical Transcription Course has been designed to be fully compatible with tablets and smartphones. Career path Having this Medical Writing, Clinical Coding & Medical Transcription various expertise will increase the value of your CV and open you up to multiple job sectors.

ICT03: Assuring Data Integrity in Clinical Research
By Zenosis
Pharmaceutical, biotechnology and medical device companies and clinical researchers need to assure regulatory authorities of the reliability of the data that they generate during product development and testing – that is, to demonstrate data integrity. Practices that provide assurance of data integrity in clinical research are required by law and/or established as expectations in regulatory guidance. The data are reviewed in regulatory applications or during regulatory inspections of clinical trial sponsor and investigational sites. Inadequacies of data integrity are frequently reported by inspectors and result in regulatory actions against the organizations or individuals concerned. This course explains the requirements and describes principles and practices that should be followed by trial sponsors, investigators and other clinical research personnel to assure regulators of data integrity.

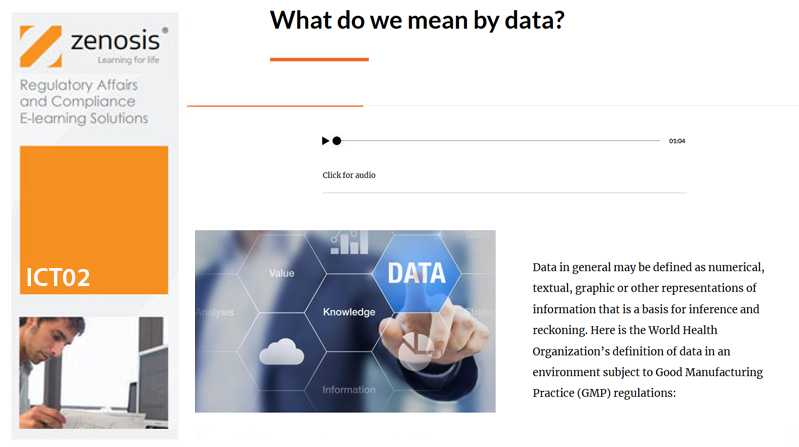
ICT02: Assuring Data Integrity in the Manufacture of Medicinal Products
By Zenosis
Pharmaceutical and biotechnology companies and researchers need to assure regulatory authorities of the reliability of the data that they generate or acquire during product development and manufacturing – that is, to demonstrate data integrity. Data integrity is assessed during regulatory inspections of manufacturing and research sites. Inadequacies of data integrity are frequently reported by inspectors and result in regulatory actions against the companies or individuals concerned. Practices that assure data integrity are required by law and/or expected by regulators in the fields of nonclinical and clinical research, manufacturing and distribution, and pharmacovigilance of medicinal products. This course explains the requirements and describes principles and practices that should be followed to assure regulators and contractual partners of data integrity in the manufacture of medicinal products.
In today’s healthcare environment, maintaining high standards of professionalism is essential for allied health professionals who support patient care and wellbeing. This course offers a focused exploration of key areas such as data protection under Healthcare GDPR, workplace professionalism, and effective communication tailored specifically to the health and care sectors. It addresses critical topics including social media conduct, document control, and patient customer service, ensuring learners gain essential knowledge to uphold ethical and professional standards in their roles. Through a structured curriculum, participants will deepen their understanding of equality, diversity, and cross-cultural awareness, alongside person-centred care and decision-making processes. The course also covers infection control and safe medicine handling, providing a well-rounded foundation that supports safe, respectful, and effective care delivery. Designed for busy professionals, this entirely online programme ensures flexibility without compromising on quality, making it an ideal choice for those seeking to strengthen their professional approach within allied health settings. This Professionalism in Allied Health bundle contains the following courses : Course 01: Healthcare GDPR Course 02: Workplace Professionalism Certificate Course Course 03: Social Media for Health & Care Course 04: Document Control Course 05: Patient Customer Service Course 06: Equality, Diversity and Discrimination Course 07: Communication Skills Course 08: Cross-Cultural Awareness Training Course 09: Person-Centred Care Course 10: Decision Making and Critical Thinking Course 11: Infection Control & Medicine Handling Key Features: CPD Certified Instant e-certificate and hard copy dispatch by next working day Fully online, interactive course with audio voiceover Developed by qualified professionals in the field Self-paced learning and laptop, tablet, smartphone-friendly 24/7 Learning Assistance Discounts on bulk purchases Here are brief descriptions for each course in the "Professionalism in Allied Health" bundle: Healthcare GDPR: This course provides an overview of the General Data Protection Regulations (GDPR) in the context of healthcare settings, covering topics such as lawful basis for data processing, responsibilities, obligations, and handling electronic medical records. Workplace Professionalism Certificate Course: Designed to enhance professionalism in the workplace, this course covers strategies for positioning oneself professionally, improving professional image, expanding skills, effective communication, and building professional relationships. Social Media for Health & Care: Explore the intersection of social media and healthcare, understanding its impact on communication, big data, medical content creation, participatory health, hospital use, health behavior change, and applications in the healthcare sector. Document Control: Gain insight into managing documents effectively throughout their lifecycle, covering principles of document control, strategies, quality assurance, project document control, and electronic document management systems. Patient Customer Service: Learn the essentials of providing excellent customer service in medical settings, including effective training, communication skills, scheduling, medical terminology, filing systems, and handling difficult situations. Equality, Diversity and Discrimination: Understand concepts of equality, diversity, and discrimination, exploring legislation, integration into policy, human rights, promoting diversity, equality analysis, stereotypes, prejudice, discrimination, bias, and affirmative action. Communication Skills: Enhance basic and advanced communication skills, including telephone etiquette, body language, and effective communication strategies for various contexts. Cross-Cultural Awareness Training: Develop cultural competence through understanding cross-cultural communication, corporate awareness, design, competency, values, and working with culturally diverse teams. Person-Centred Care: Implement person-centered care by understanding individual history, preferences, needs, environmental factors, promoting values, and recognizing signs of discomfort or distress. Decision Making and Critical Thinking: Develop critical thinking skills to evaluate claims, identify benefits and barriers, problem-solve, and make decisions effectively. Infection Control & Medicine Handling: Ensure safe administration, storage, distribution, and disposal of medicines, while also addressing infection control measures and potential risks associated with handling medications. Learning Outcomes: Understand GDPR regulations in healthcare settings for data protection. Demonstrate professionalism in various workplace scenarios and environments. Apply social media strategies effectively within healthcare and allied fields. Implement document control procedures to maintain accuracy and confidentiality. Deliver exceptional customer service tailored to patient needs and expectations. Foster inclusivity and combat discrimination through awareness and understanding. Accreditation All of our courses, including the Professionalism in Allied Health Bundle are fully accredited, providing you with up-to-date skills and knowledge and helping you to become more competent and effective in Financial Fraud Detection. Certification Once you've successfully completed your Professionalism in Allied Health Bundle, you will immediately be sent your digital certificates. Also, you can have your printed certificate delivered by post (shipping cost £3.99). Our Professionalism in Allied Health certification have no expiry dates, although we recommend renewing them every 12 months. CPD 110 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Healthcare professionals seeking enhanced professionalism and compliance knowledge. Allied health workers aiming to improve communication and patient care skills. Individuals interested in advancing their career prospects in healthcare settings. Students pursuing allied health studies or related disciplines. Professionals aiming to navigate diverse workplace environments with proficiency. Requirements There are no formal entry requirements for the course, with enrollment open to anyone! Career path Healthcare Administrator Patient Care Coordinator Medical Records Technician Health Education Specialist Clinical Research Coordinator Pharmaceutical Sales Representative Certificates Digital certificate Digital certificate - Included Once you've successfully completed your course, you will immediately be sent a FREE digital certificate. Hard copy certificate Hard copy certificate - Included Also, you can have your FREE printed certificate delivered by post (shipping cost £3.99 in the UK). For all international addresses outside of the United Kingdom, the delivery fee for a hardcopy certificate will be only £10. Our certifications have no expiry dates, although we do recommend that you renew them every 12 months.

Master the art of sterile compounding with comprehensive online training. Learn essential techniques such as hand washing, garbing, and cleaning laminar flow hoods. Gain proficiency in compounding pharmacy math and sterile pharmaceutical product preparation. Ideal for pharmacy professionals seeking to enhance their skills and advance their careers. Start your training today!

Pharmacy Assistant & Technician - Training
By Training Tale
This Pharmacy Assistant & Technician course is customised only for you! Do you want to advance your career in the healthcare industry? Or, are you looking for a way to contribute to community health care without performing clinical duties? If you're considering a career in the pharmaceutical industry, then you've come to the right place. This Pharmacy Assistant & Technician course teaches you everything you need to know to become a Pharmacy Technician or Pharmacy Technician Assistant and provide support and stability to your team. By enrolling on our Pharmacy Assistant & Technician course, you will become familiar with the roles and responsibilities of a Pharmacy Assistant or Technician. This comprehensive Pharmacy Assistant & Technician course will teach you the fundamental techniques of a Pharmacy worker and how to successfully counsel and diagnose a patient. You will also gain experience in the practice of prescribing and dispensing medication, as well as stock control and inventory management. Along with this, you will gain knowledge of medical health and safety standards and security protocols associated with legal drugs. Enrol in this Pharmacy Assistant & Technician course now and start your career in the UK's healthcare sector. Learning Outcomes After completing Pharmacy Assistant & Technician course, the learner will be able to: Understand the requirements needed to be a Pharmacy Technician or Assistant. Know how to counsel or communicate with patients and customers. Become aware of your position in the UK Healthcare infrastructure. Gain the ability to prescribe and dispense medication swiftly. Know to control and manage a medical inventory. Gain a solid understanding of the Standard Operating Procedures in a medical environment. Gain the skills to perform a full medical assessment on a patient. Why Choose Pharmacy Assistant & Technician Course from Us Self-paced course, access available from anywhere. Easy to understand, high-quality study materials. Pharmacy Assistant & Technician Course developed by industry experts. MCQ quiz after each module to assess your learning. Automated and instant assessment results. 24/7 support via live chat, phone call or email. Free PDF certificate as soon as completing this course. ***Pharmacy Assistant & Technician Bundle Course Course 01: Pharmacy Assistant & Technician Course 02: Healthcare Management Training Course 03: Level 3 Diploma in Healthcare Support Course 04: Level 2 Award in Nutrition and Health Course 05: Ambulance Care Assistant Course 06: Level 3 Award in Health and Social Care Course Course 07: Level 6 Diploma in Health and Social Care Management Course 08: Martial Arts First Aid Course 09: Paediatric First Aid Course 10: Safeguarding Vulnerable Adults Training Course 11: Nursing & Prescribing ***Other Benefits of this Bundle Course Free 11 PDF Certificate Access to Content - Lifetime Exam Fee - Totally Free Free Retake Exam [ Note: Free PDF certificate will provide as soon as completing the Pharmacy Assistant & Technician course] Course Curriculum of *** Pharmacy Assistant & Technician *** Module 1: An Overview of Pharmacy Assistant and Technician Module 2: Understanding the Pharmacy Assistant Patient Counselling Guide Module 3: Understanding Communication in Pharmacy Settings Module 4: Understanding the Pharmacy Team and Practices Module 5: Understanding Prescription and Dispensing in Pharmacies Module 6: Understanding Dispensing Methods, EPS, Minimising Dispensing Errors in Pharmacies Module 7: Understanding Inventory Control and Management in Pharmacies Module 8: Understanding Standard Operating Procedures (SOPs) Module 9: Understanding Health and Safety Risks Assessment and Pharmaceutical Terminology --------------------- Assessment Method After completing each module of the Pharmacy Assistant & Technician Course, you will find automated MCQ quizzes. To unlock the next module, you need to complete the quiz task and get at least 60% marks. Certification After completing the MCQ/Assignment assessment for this course, you will be entitled to a Certificate of Completion from Training Tale. The certificate is in PDF format, which is completely free to download. A printed version is also available upon request. It will also be sent to you through a courier for £13.99. Who is this course for? This Pharmacy Assistant & Technician course is ideal for those interested in becoming pharmacy technicians or those looking to make a career in the medical field. Requirements There are no specific requirements for Pharmacy Assistant & Technician course because it does not require any advanced knowledge or skills. Students who intend to enrol in this Pharmacy Assistant & Technician course must meet the following requirements: Good command of the English language Must be vivacious and self-driven Basic computer knowledge A minimum of 16 years of age is required Career path This Pharmacy Assistant & Technician qualification is beneficial for any healthcare profession or career from any industry you are in, such as: Pharmacist's Assistant Pharmacy Technician Hospital Clerk Pharmacist Certificates Certificate of completion Digital certificate - Included

Search By Location
- Pharmaceutical Marketing Courses in London
- Pharmaceutical Marketing Courses in Birmingham
- Pharmaceutical Marketing Courses in Glasgow
- Pharmaceutical Marketing Courses in Liverpool
- Pharmaceutical Marketing Courses in Bristol
- Pharmaceutical Marketing Courses in Manchester
- Pharmaceutical Marketing Courses in Sheffield
- Pharmaceutical Marketing Courses in Leeds
- Pharmaceutical Marketing Courses in Edinburgh
- Pharmaceutical Marketing Courses in Leicester
- Pharmaceutical Marketing Courses in Coventry
- Pharmaceutical Marketing Courses in Bradford
- Pharmaceutical Marketing Courses in Cardiff
- Pharmaceutical Marketing Courses in Belfast
- Pharmaceutical Marketing Courses in Nottingham