- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
11303 Courses
ESS01: Essentials of EU and US Regulatory Affairs for Human Medicinal Products
By Zenosis
This foundation-level module is the ideal introduction for new entrants to the field of pharmaceutical regulatory affairs and compliance. It describes the principal requirements that must be satisfied to gain and maintain approval to market medicinal products in the USA and Europe. The legal framework and the roles of major players in regulation are presented. The life-cycle of a drug is outlined. The various procedures available for assessment and approval of products are described and their requirements outlined. Obligations to be fulfilled after marketing approval are discussed.

Family Law, Family Support Worker and Safeguarding Diploma Transform your career with our Family Law, Family Support Worker and Safeguarding Diploma. Become the Family Support Worker that families trust. Drive change as an informed and skilled Family Support Worker. Learning Outcomes: Master key Family Law principles relevant to a Family Support Worker. Address discrimination effectively as a Family Support Worker. Develop essential qualities intrinsic to an effective Family Support Worker. Deliver crisis support with a Family Support Worker's skill set. Apply safeguarding laws in your role as a Family Support Worker. More Benefits: LIFETIME access Device Compatibility Free Workplace Management Toolkit Key Modules from Family Law, Family Support Worker and Safeguarding Diploma: Introduction to Family Law: Acquire Family Law basics essential for a Family Support Worker's role. Discrimination in Family Law: Identify and combat discrimination through a Family Support Worker's legal understanding. Qualities of a Family Support Worker: Cultivate the character and competencies necessary to excel as a Family Support Worker. Supporting Families in Crisis: Equip yourself with crisis management techniques tailored for a Family Support Worker. Laws and Guidance on Safeguarding: Integrate safeguarding laws and guidance into your practices as a Family Support Worker. Safeguarding of Vulnerable Adults: Ensure the well-being of vulnerable adults by applying principles aligned with a Family Support Worker's responsibilities.

AITT Instructor Training
By Mha Training Ltd - Aitt | Ipaf | Iosh | First Aid | Mental Health First Aid
Instructor training enables companies to have their own AITT Forklift Instructor. On completion they are able to instruct and examine operators on company premises. Also it could be an opportunity for an individual to embark upon a rewarding career as a recognised AITT instructor. The course complies with the approved code of practice issued by the Health & Safety Executive. We offer the AITT Instructor training course at our training centre in Warrington, Cheshire. We use all the best tools and equipment to assist trainee development. Our Instructor’s Mike Hammett and Stephen McCann have a lot of experience in this course, both have very good success rates and offer alot of after care too! Once an Instructor has passed their AITT Instructor training course they can always come back and receive professional advice. We always go the extra mile! AITT Accredited Novice Course: The Instructor training course caters for candidates seeking to become an AITT Registered Instructor. Previous fork lift experience is strongly recommended and candidates must have a current counterbalance certificate dated within 36 months prior to the course start date. Refresher courses are available prior to the instructor course extending the duration by one day to 11 days. Objectives: On successful completion of the course the candidate will be qualified to teach and train on all Industrial Counterbalance and Reach trucks for which they are certificated to use as operators. Target Group: The employer should carefully select the correct person for the job as an instructor. They should be literate and numerate with good presentation skills. The AITT recommend that candidates have a minimum of 12 months operating experience before attending the course. During the course candidates will be progressively assessed in all key areas. Candidates therefore must have a good knowledge of each subject and are provided with some excellent materials to assist them on completion of the course. AITT Instructor Training Course Duration: 3 or 5 days for Re-qualification or Re-Registration courses. 5 days for Assimilation Courses. 10-12 days for the Novice AITT instructor training course. Contents: Principles of instruction. Instructional techniques. HASAWA 1974/PUWER 1998/LOLER 1998/L117. Setting up courses. Administering the tests etc. All original documentation supplied by examining body and HSE. Prices are available on request and should you require any further information please do not hesitate to contact us. We also offer In-House Instructor training to suit companies needs and these are of five day durations, please contact for further details. Please feel free to download our Course Syllabus’s below and decide which course best meets your needs. See Mike at work demonstrating a lesson of De-stacking from High Level. In-House Courses: These courses are aimed at companies wishing to use their own Instructors to train staff. IN-HOUSE BASIC INSTRUCTOR COURSE PDF AITT Instructor Training Courses: On completion of these courses candidates will be registered as an AITT Instructor and be able to train on anything they are currently qualified to operate. Courses vary depending on experience and current qualifications so please have a look at the following courses to see which suits best. More information is available at www.aitt.co.uk.

Internal Workplace Mediation Skills Course (5 days)
By Buon Consultancy
Workplace Mediation

SUB11: The Decentralised Procedure (DCP)
By Zenosis
This module describes the roles of the various players in the procedure, the sequence and duration of the stages involved, and the requirements on content, format and timing of submissions. It discusses the special issues that apply to generic products in the DCP.
SUB03: The Mutual Recognition Procedure (MRP)
By Zenosis
his module describes the roles of the various players in the procedure, the sequence and duration of the stages involved, and the requirements on content, format and timing of submissions. It discusses the special issues that apply to generic products in the MRP.
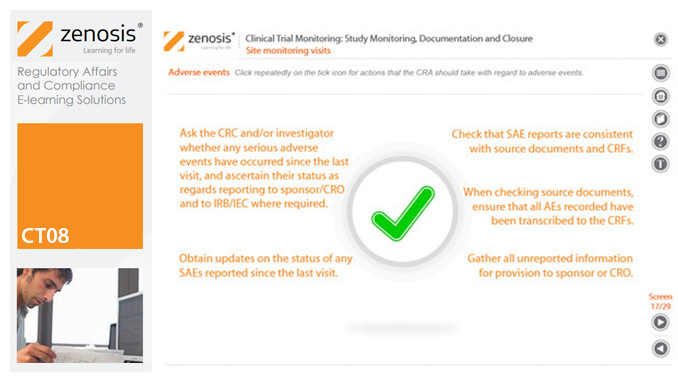
CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
By Zenosis
The sponsor of a clinical trial must arrange for it to be monitored throughout its duration to ensure that the rights and wellbeing of subjects are protected, the trial data are accurate, complete and verified from source documents, and the conduct of the trial complies with the study protocol, Good Clinical Practice and regulatory requirements. In this module we describe how a Clinical Research Associate (CRA) monitors an ongoing trial to its conclusion.
24-Hour Knowledge Knockdown! Prices Reduced Like Never Before Want to ensure business compliance and risk management excellence? 92% of UK organisations prioritise compliance and risk management for sustainable growth. So, it's understandable that compliance and risk management are essential for businesses to mitigate legal and operational risks. Hence, our Compliance bundle equips professionals with the skills and knowledge to navigate and implement effective compliance and risk management strategies. With a single payment, you will gain access to Compliance & Risk Management course, including 10 premium courses, a QLS Endorsed Hardcopy certificate and 11 PDF certificates for Absolutely free. This Compliance & Risk Management Bundle Package includes: Course 01: Diploma in Compliance Management at QLS Level 5 10 Premium Additional CPD QS Accredited Courses - Course 01: Conflict Management Training Course 02: Mastering Organizational Chaos Course Course 03: Workplace Team Networking Diploma Course 04: Legal Operations Consultant Course 05: Decision-Making and Critical Thinking Training Course 06: Unconscious Bias Training Course Course 07: Cross-Cultural Awareness Training Course 08: Workplace Stress Management Diploma Course 09: Workplace Productivity Course Course 10: Corporate Risk And Crisis Management So, join in this journey towards a fulfilling career, where your Compliance and Risk Management knowledge will be in high demand. Learning Outcomes Master Compliance and Risk Management principles and strategies. Develop conflict resolution skills for effective Risk Management. Mitigate risks effectively with thorough Risk Management knowledge. Promote diversity and inclusivity through Risk Management initiatives. Foster cross-cultural awareness for better Compliance Management. Why Choose Our Bundle? Get a Free QLS Endorsed Certificate upon completion of Compliance course Get a free student ID card with Compliance Training Get instant access to this Compliance course. Learn Compliance from anywhere in the world The Compliance is affordable and simple to understand The Compliance is an entirely online, interactive lesson with voiceover audio Lifetime access to the Compliance course materials The Compliance comes with 24/7 tutor support Start your learning journey straightaway! This Compliance Management's curriculum has been designed by Compliance Management experts with years of Compliance Management experience behind them. The Compliance Management course is extremely dynamic and well-paced to help you understand Compliance Management with ease. Assessment Process You will be entitled to claim a certificate endorsed by the Quality Licence Scheme after you have completed all of the Diploma in Compliance Management at QLS Level 5 exams. CPD 255 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Compliance & Risk Management Requirements Compliance & Risk Management Career path Compliance & Risk Management Certificates CPD QS Accredited PDF Certificate Digital certificate - Included Diploma in Compliance Management at QLS Level 5 Hard copy certificate - Included

SAM04: Marketing of Prescription Drugs in the USA – Interactions with Healthcare Professionals
By Zenosis
The heaviest legal penalties imposed on drug companies concern interactions with healthcare professionals in the context of prescription drug marketing, notably for violations of the Anti-Kickback Statute and the False Claims Act. Monetary penalties have amounted to billions of dollars in some cases.

SAM02: Regulatory Requirements and Guidance on Advertising and Promotion of Prescription Drugs in the USA
By Zenosis
In this course we explain how to advertise and promote prescription drugs in various media, whether to healthcare professionals or consumers, in compliance with legal requirements and guidance from the FDA.

Search By Location
- Safety Courses in London
- Safety Courses in Birmingham
- Safety Courses in Glasgow
- Safety Courses in Liverpool
- Safety Courses in Bristol
- Safety Courses in Manchester
- Safety Courses in Sheffield
- Safety Courses in Leeds
- Safety Courses in Edinburgh
- Safety Courses in Leicester
- Safety Courses in Coventry
- Safety Courses in Bradford
- Safety Courses in Cardiff
- Safety Courses in Belfast
- Safety Courses in Nottingham