- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
11302 Courses
VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
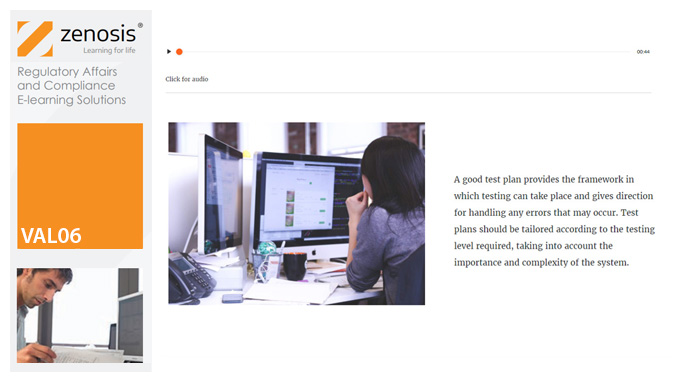
VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

VAL04: Operational and Performance Qualification
By Zenosis
Having undergone Installation Qualification, before equipment can be used routinely in production, it needs to undergo Operational Qualification (OQ) and Performance Qualification (PQ). This module describes OQ and PQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
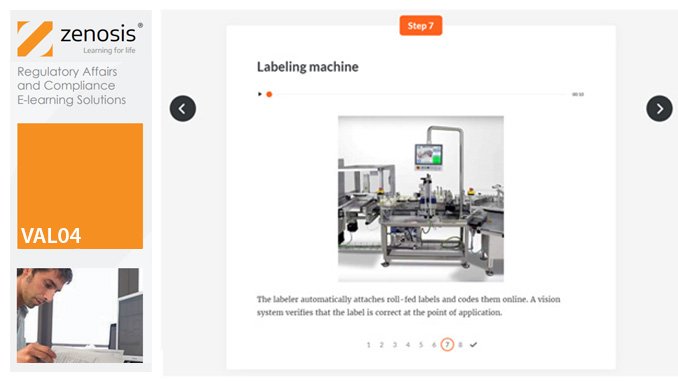
VAL03: Commissioning and Installation Qualification
By Zenosis
Before equipment can be used routinely in production, it must first be commissioned and, if necessary, undergo Installation Qualification (IQ). This module describes commissioning and IQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
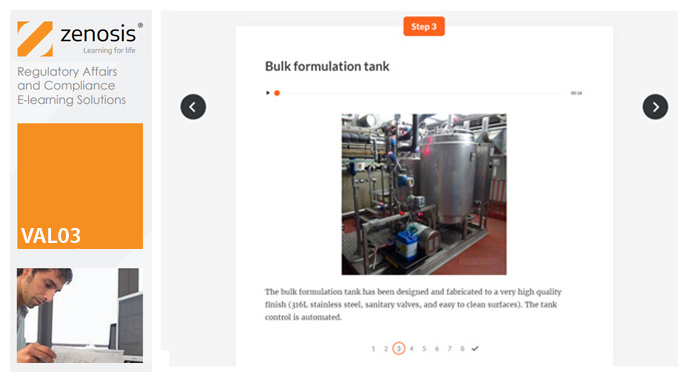
VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
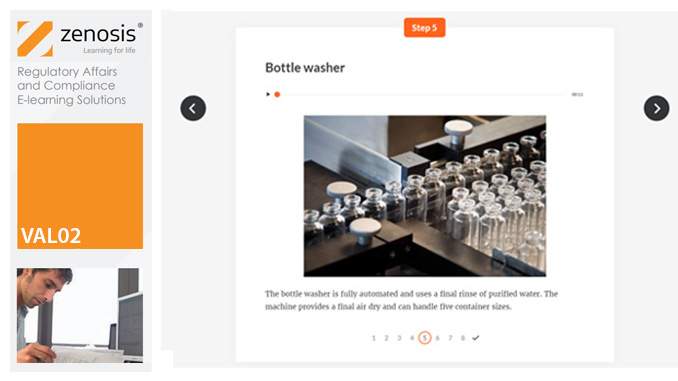
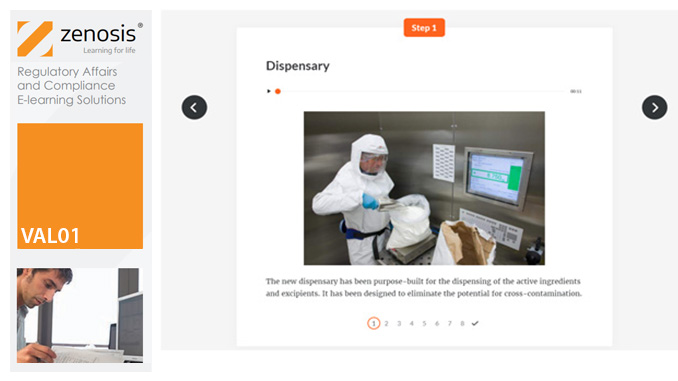
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
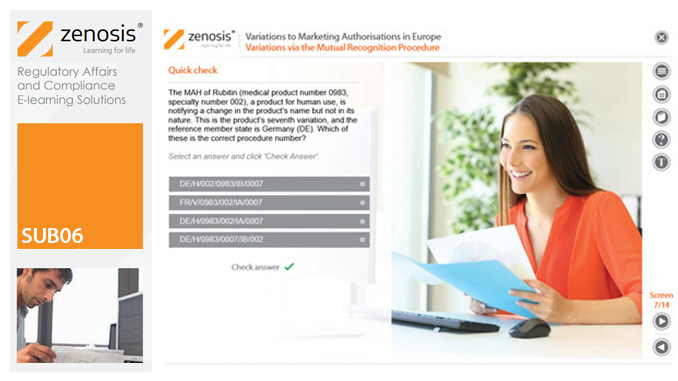
SUB06: Variations to Marketing Authorisations in Europe
By Zenosis
Changes to the terms of marketing authorisations for medicinal products, called variations in Europe, must be notified to or approved by the relevant regulatory authorities. Variations include changes to the composition of products, their manufacturing processes, the way they are used, or the indications for which they are authorised. Common approaches are adopted within the European Economic Area to variations to marketing authorisations approved through the Centralised, Decentralised or Mutual Recognition Procedures. Recent legislation has substantially modified the regulatory requirements and extended them to purely national authorisations by member states. This module, which is fully up to date with the new legislation, covers the classification of variations into their several types and the regulatory requirements, guidance and procedures to be followed for each type.
ESS01: Essentials of EU and US Regulatory Affairs for Human Medicinal Products
By Zenosis
This foundation-level module is the ideal introduction for new entrants to the field of pharmaceutical regulatory affairs and compliance. It describes the principal requirements that must be satisfied to gain and maintain approval to market medicinal products in the USA and Europe. The legal framework and the roles of major players in regulation are presented. The life-cycle of a drug is outlined. The various procedures available for assessment and approval of products are described and their requirements outlined. Obligations to be fulfilled after marketing approval are discussed.

Family Law, Family Support Worker and Safeguarding Diploma Transform your career with our Family Law, Family Support Worker and Safeguarding Diploma. Become the Family Support Worker that families trust. Drive change as an informed and skilled Family Support Worker. Learning Outcomes: Master key Family Law principles relevant to a Family Support Worker. Address discrimination effectively as a Family Support Worker. Develop essential qualities intrinsic to an effective Family Support Worker. Deliver crisis support with a Family Support Worker's skill set. Apply safeguarding laws in your role as a Family Support Worker. More Benefits: LIFETIME access Device Compatibility Free Workplace Management Toolkit Key Modules from Family Law, Family Support Worker and Safeguarding Diploma: Introduction to Family Law: Acquire Family Law basics essential for a Family Support Worker's role. Discrimination in Family Law: Identify and combat discrimination through a Family Support Worker's legal understanding. Qualities of a Family Support Worker: Cultivate the character and competencies necessary to excel as a Family Support Worker. Supporting Families in Crisis: Equip yourself with crisis management techniques tailored for a Family Support Worker. Laws and Guidance on Safeguarding: Integrate safeguarding laws and guidance into your practices as a Family Support Worker. Safeguarding of Vulnerable Adults: Ensure the well-being of vulnerable adults by applying principles aligned with a Family Support Worker's responsibilities.

AITT Instructor Training
By Mha Training Ltd - Aitt | Ipaf | Iosh | First Aid | Mental Health First Aid
Instructor training enables companies to have their own AITT Forklift Instructor. On completion they are able to instruct and examine operators on company premises. Also it could be an opportunity for an individual to embark upon a rewarding career as a recognised AITT instructor. The course complies with the approved code of practice issued by the Health & Safety Executive. We offer the AITT Instructor training course at our training centre in Warrington, Cheshire. We use all the best tools and equipment to assist trainee development. Our Instructor’s Mike Hammett and Stephen McCann have a lot of experience in this course, both have very good success rates and offer alot of after care too! Once an Instructor has passed their AITT Instructor training course they can always come back and receive professional advice. We always go the extra mile! AITT Accredited Novice Course: The Instructor training course caters for candidates seeking to become an AITT Registered Instructor. Previous fork lift experience is strongly recommended and candidates must have a current counterbalance certificate dated within 36 months prior to the course start date. Refresher courses are available prior to the instructor course extending the duration by one day to 11 days. Objectives: On successful completion of the course the candidate will be qualified to teach and train on all Industrial Counterbalance and Reach trucks for which they are certificated to use as operators. Target Group: The employer should carefully select the correct person for the job as an instructor. They should be literate and numerate with good presentation skills. The AITT recommend that candidates have a minimum of 12 months operating experience before attending the course. During the course candidates will be progressively assessed in all key areas. Candidates therefore must have a good knowledge of each subject and are provided with some excellent materials to assist them on completion of the course. AITT Instructor Training Course Duration: 3 or 5 days for Re-qualification or Re-Registration courses. 5 days for Assimilation Courses. 10-12 days for the Novice AITT instructor training course. Contents: Principles of instruction. Instructional techniques. HASAWA 1974/PUWER 1998/LOLER 1998/L117. Setting up courses. Administering the tests etc. All original documentation supplied by examining body and HSE. Prices are available on request and should you require any further information please do not hesitate to contact us. We also offer In-House Instructor training to suit companies needs and these are of five day durations, please contact for further details. Please feel free to download our Course Syllabus’s below and decide which course best meets your needs. See Mike at work demonstrating a lesson of De-stacking from High Level. In-House Courses: These courses are aimed at companies wishing to use their own Instructors to train staff. IN-HOUSE BASIC INSTRUCTOR COURSE PDF AITT Instructor Training Courses: On completion of these courses candidates will be registered as an AITT Instructor and be able to train on anything they are currently qualified to operate. Courses vary depending on experience and current qualifications so please have a look at the following courses to see which suits best. More information is available at www.aitt.co.uk.

Search By Location
- Safety Courses in London
- Safety Courses in Birmingham
- Safety Courses in Glasgow
- Safety Courses in Liverpool
- Safety Courses in Bristol
- Safety Courses in Manchester
- Safety Courses in Sheffield
- Safety Courses in Leeds
- Safety Courses in Edinburgh
- Safety Courses in Leicester
- Safety Courses in Coventry
- Safety Courses in Bradford
- Safety Courses in Cardiff
- Safety Courses in Belfast
- Safety Courses in Nottingham