- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Courses in London
We couldn't find any listings for your search. Explore our online options and related educators below to see if they help you.
Know someone teaching this? Help them become an Educator on Cademy.
Online Options
Show all 31766Certified Data Centre Environmental Sustainability Specialist (CDESS)
By Nexus Human
Duration 5 Days 30 CPD hours This course is intended for The primary audience for this course is any IT, facilities or data centre professional who works in and around the data centre and has the responsibility to achieve and improve efficiency and environmental sustainability, whilst maintaining the availability and manageability of the data centre. Overview After completion of the course the participant will be able to: Understand the impact of data centres on the environment Describe the various environmental/energy management standards Understand the purpose and goals of the legally binding international treaties on climate change Implement various sustainable performance metrics and how to use them in the data centre environment Manage data centre environmental sustainability using international standards Set up the measurement, monitoring and reporting of energy usage Use power efficiency indicators in a variety of data centre designs Use best practices for energy savings in the electrical infrastructure and in the mechanical (cooling) infrastructure Use best practices for energy savings for the ICT equipment and data storage Understand the importance of water management and waste management Understand the different ways to use sustainable energy in the data centre Get practical tips and innovative ideas to make a data centre more sustainable The CDESS© course is aimed at providing knowledge of the standards and guidelines related to environmental sustainability, and how to move your data centre (existing or new) to a more environmentally sustainable design and operations. Impact of Data Centres on the Environment Predictions in 2010 Current situation Outlook and commitments What is Environmental Sustainability The importance of sustainability Senior management commitment Environmental sustainability framework Sustainability policies Performance standards and metrics Information policies Transparency Awareness Service charging models Environmental Management Environmental sustainability framework (ISO 14001) Standards and guidelines ? ISO 50001 / ISO 30134 Measurement and categories Baselining Trend analysis Reporting Power Effiðciency Indicators Various eðfficiency indicators Power Usage Effectiveness (PUE) PUE measurement levels Factors affecting PUE Measurement points and intervals PUE in mixed source environments Measuring PUE in a mixed-use building PUE reporting Impact of PUE after optimising IT load Electrical Energy Savings (Electrical) Identifying the starting point for saving energy Sizing of power DC power Generators UPS systems Power Factor (PF) Energy savings on lighting Electrical Energy Savings (Mechanical) Energy savings on the cooling infrastructure Temperature and humidity setpoints Various energy eðcient cooling technologies Energy savings on the airflow Liquid cooling Energy reusage PUE, ERE/ERF and Control Volume Electrical Energy Savings (ICT) Procurement IT equipment energy eðfficiency ITEEsv, SMPE, SMPO IT equipment utilisation Server virtualisation Open compute project Electrical Energy Savings (Data Storage) Data management Data storage management Data storage equipment effiðciency Water Management Water Usage Effectiveness (WUE) Improving WUE Water usage at the power generation source Energy Water Intensity Factor (EWIF) Waste Management Waste management policies Life-cycle assessment (Cradle to the grave) 3 R?s for waste management Reduce Reuse Second-hand market Recycle Sustainable Energy Usage Sustainable energy sources Power purchase agreements Energy attribute certificates Renewable Energy Factor (REF) Matching renewable energy supply and demand Sustainable energy storage Carbon trading Automated Environmental Management Systems Use of AI and machine learning Load migration Data Centre Infrastructure Management (DCIM) solutions

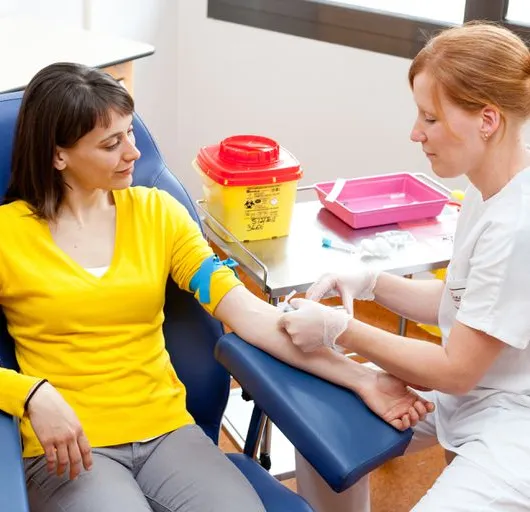
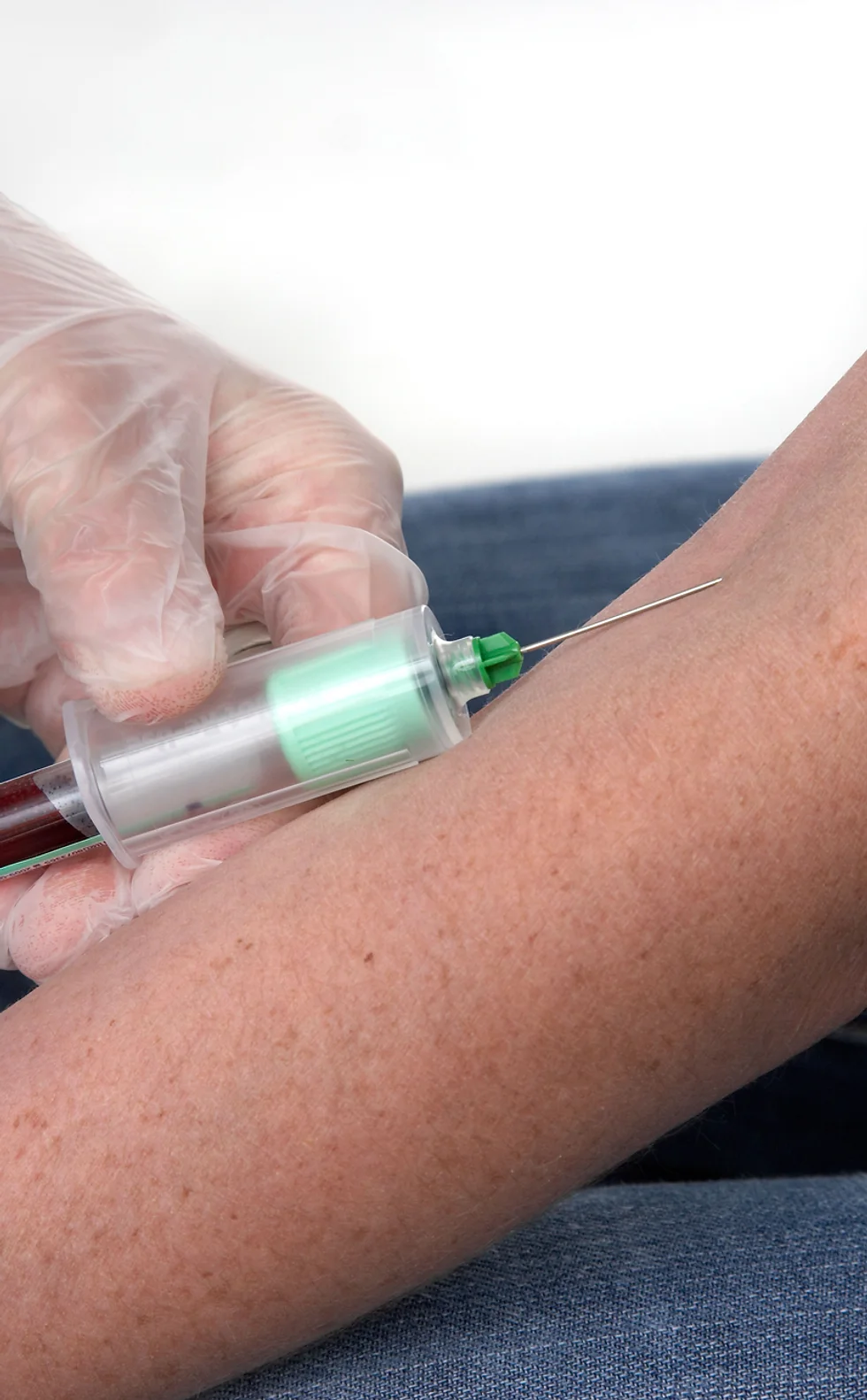
THIS COURSE PACKAGE INCLUDES: 1: INTRODUCTION TO PHLEBOTOMY COURSE (GPT003) - Level 3 (Ireland Level 5) 2: ADVANCED PHLEBOTOMY COURSE (GPT005) - Level 4 (Ireland Level 6) 3: GEOPACE COMPETENCY CERTIFICATE - CPD Certified (optional with Virtual Classroom) Learn how to take blood ... train as a Phlebotomist FAST-TRACK YOUR PHLEBOTOMY TRAINING WITH OUR COMPLETE TRAINING PACKAGE 20% off - Multi-Course Discount Cover all stages from beginner through to Level 4 Available as Classroom or Virtual Classroom Complete your beginner to advanced training in 2 days Awards 2 accredited qualifications - Introduction to Phlebotomy and Advanced Phlebotomy qualifications Both courses are dually accredited (OCN & CPD) Geopace Certificate of Competency included with classroom attendance or available as an option when booking virtual classroom Covers all steps up to live blood draw Learn advanced skills and techniques Virtual Classroom options include comprehensive Practise@Home Training Kits (yours to keep) Basic understanding of English language required OPEN TO ALL APPLICANTS
Introduction to Good Manufacturing Practice
By Research Quality Association
Course Information This course offers foundational guidance and practical support tailored for individuals operating within Good Manufacturing Practice (GMP) frameworks. Explore the fundamental prerequisites of a pharmaceutical quality system (PQS) and delve into the application of quality risk management (QRM) principles, aligning with current regulations and guidance. Gain insights into pivotal aspects such as requirements, roles, and responsibilities, encompassing change control, document management, and key documentation essential for effective implementation of GMP with a focus on regulatory inspections and common findings. Is this course for you? Ideal for professionals engaged in GMP across various sectors, including: Research and Development (R&D) Contract Manufacturing Organisations Manufacturing Units Quality Control (QC) Laboratories Auditing Roles. What will you learn? Event objectives - by the end of the course, delegates shall: Have an awareness of the basic requirements of GMP Be aware of UK and EU GMP Rules and Guidance and relevant publications Understand the roles and responsibilities associated with GMP Be able to contribute to and maintain quality documentation Have a basic understanding of product lifecycle and manufacturing Understand the requirements of GMP in the QC laboratory context Have a basic understanding of risk management and mitigation principles Understand the need for quality systems and quality assurance activities Be aware of common regulatory findings. Learning outcomes: delegates will be able to: Implement their role within GMP with confidence and knowledge of the principle requirements Contribute effectively to the GMP quality system and their organisation’s compliance Comprehend where their organisation’s activities sit within the larger GMP arena Know where to seek further information within the published rules and guidance, UK Legislation, European Commission Directives, ICH Guidance and other relevant publications, as well as via the internet. Tutors Tutors will be comprised of (click the photos for biographies): Louise Handy Director, Handy Consulting Ltd Programme Please note timings may be subject to alteration. Day 1 09:30 Introductions and Scope of the Course Understand the group requirements and the tutor's background and experience. 09:45 Background and Regulatory Environment Setting the scene, understanding the context, key legislation. 10:30 Principles of GMP Key points and requirements. 11:15 Break 11:30 Personnel and Responsibilities Management and staff, duties and accountabilities. 12:00 Overview of GMP Manufacturing Basics of the product life cycle. 12:30 Lunch 13:15 Risk Management Workshop Practical exploration of risk and mitigation activities. 14:30 QC Laboratories Activities and practicalities. 15:15 Break 15:30 Compliance Quality Assurance and Self Inspection. 16:15 Question Time A chance for questions on the practicalities of GMP. 16:30 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Learn

App in a day
By Nexus Human
Duration 1 Days 6 CPD hours This course is intended for Looking for a way to extend your business operations? Look no further than the App in a Day workshop! In this one day course you will learn to build a custom, secure business application that you can share across your organise and will run across multiple platforms including tablets and mobile devices. Power Platform is a secure and scalable platform for building your own applications. In this course, you will learn how to build an application from the ground up by building the required UX, Backend, and frontend. All this without writing any code. Overview Some of the applications of the Power Platform you will learn to build will allow you to potentially: A) Share information with trusted colleagues and associates B) Monitor and manage your business operations C) Monitor and manage your customers D) Share business news on social media E) Share business photos and footage This course will teach you how to build your own application that is completely secure and private. Power Apps Canvas App Connect to data sources and filter results based on specified criteria Work with screens and navigation Use controls, properties, formulas, and actions to customize the user experience Display the logged in user?s name Configure app settings Save and share an app Run an app on a mobile device Microsoft Dataverse Create and customize a custom table Use the Form control Save data into the Microsoft Dataverse using the Form control Microsoft Dataverse Create a standalone Model-driven app. Customize forms for the Model-driven app Use a Business Process Flow to guide users through a process Microsoft Dataverse Create a flow that is triggered when a new Microsoft Dataverse row is created Automate sending approval requests Customize the approval based on the Microsoft Dataverse row Use the Approval centre

Excel - Automating Excel with Macros and Analysis Tools
By Nexus Human
Duration 1 Days 6 CPD hours This course is intended for To ensure success, students should have completed Excel Essentials and Excel Functions Including Pivot Tables and Lookups or have the equivalent knowledge and experience. Overview Upon successful completion of this course, students will be able to enhance productivity and efficiency by streamlining workflow, collaborate with others, and audit and analyse data. This course is designed for students desiring to gain skills necessary to create macros, collaborate with others, audit and analyse data, incorporate multiple data sources, and import data. Working with Multiple Worksheets and Workbooks Working with Named Ranges Link Cells Across Worksheets and Workbooks Use 3D References to Calculate Across Worksheets Consolidate Data Use Formula Auditing and Error Checking Reveal Formulas Trace Cell Precedents and Dependents Locate Errors in Formulas Watch and Evaluate Formulas Reviewing and Protecting Workbooks Control Data Entry via Data Validation Protect Workbook Access Protect Worksheets and Cell Content Add and Edit Comments Prepare a Workbook for Distribution Modify Excel's Default Settings Using Macros to Automate Workbook Functionality Create Macros via Recording Run Macros via Buttons and Shortcuts Assign Macros to the Quick Access Toolbar and Ribbon Assign Macros to Objects View Macro Code Forecasting and Analysis Data Use Conditional Formatting to Highlight, Sort and Filter Key Data Advanced Conditional Formatting using Formulas Create Sparklines to Visualise Data Add Trendlines to Charts to Visualise and Forecast Trends Use Data Tables and Scenarios to Project Potential Outcomes Use Goal Seek to Calculate Outcomes Forecast Data Trends Using Solver

The UK's first and only Level 4 qualification in Phlebotomy (equivalent to Ireland Level 6) FDSc (Foundation Degree Level) qualification Nationally Recognised certificate Dually accredited: Open College Network and CPD Covers both aspirated and evacuated systems Covers specialised blood collection systems & methods Classroom or Virtual Classroom learning options Comprehensive Training Kit is provided when booking our Virtual Classroom option (yours to keep) Complete your training from beginner to advanced level This course either follows on from our Introduction to Phlebotomy Course or can be combined with our introductory course as part of a course package (see below) Available to candidates who have completed (or are currently enrolled to complete) our Introduction to Phlebotomy Course or have previous phlebotomy practical experience.
An Introduction to Workplace Domestic Abuse Training (CPD Accredited)
By Safe Space Consultancy
This 1 hour on-line training seminar provides an opportunity for employers, HR managers, supervisors, and team leaders to learn how domestic abuse can impact on an employee and the business as well as the next steps to support your staff and protect your business.

Learn how to perform and read an ECG ... Nationally Recognised Qualification OCN Accredited - Level 3 (advanced level) CPD Accredited - The CPD Certification Service Introduces you to the fundamentals of setting up and operating an ECG machine Includes patient preparation Produce a valid (error free) ECG Learn and understand ECG traces Recognise recordings that require urgent attention Basic understanding of English language required OPEN TO ALL APPLICANTS VIRTUAL CLASSROOM OPTION INCLUDES COMPREHENSIVE PRACTISE@HOME ECG TRAINING KIT Final interpretation of all ECG recordings is the responsibility of a medical professional.
Search By Location
- cpd Courses in London
- cpd Courses in Birmingham
- cpd Courses in Glasgow
- cpd Courses in Liverpool
- cpd Courses in Bristol
- cpd Courses in Manchester
- cpd Courses in Sheffield
- cpd Courses in Leeds
- cpd Courses in Edinburgh
- cpd Courses in Leicester
- cpd Courses in Coventry
- cpd Courses in Bradford
- cpd Courses in Cardiff
- cpd Courses in Belfast
- cpd Courses in Nottingham

