- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
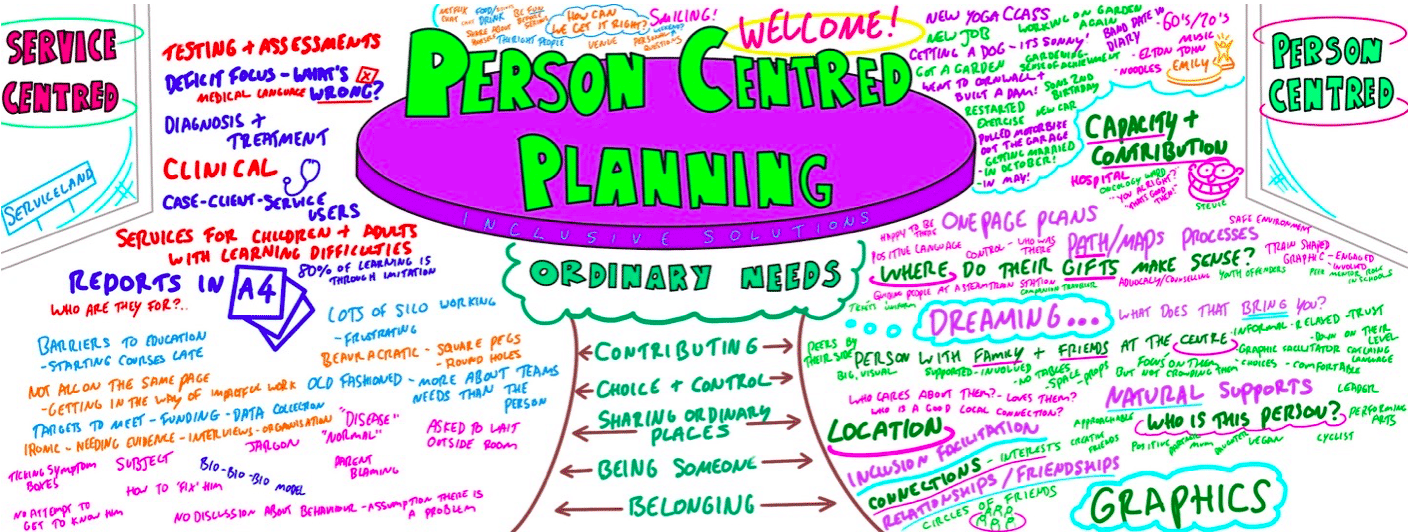
Person Centred Planning using PATH and MAPs
By Inclusive Solutions
What is Person Centred Planning? How is it different to any other kind of meeting or planning? On this day all will become clear… Give your team the opportunity to pause and reflect on what matters most to them about the work they do. The act of listening to each other creates relationship and strengthens trust and inclusion within the team – in creating a shared vision, groups of people build a sense of commitment together. They develop images of ‘the future we want to create together’, along with the values that will be important in getting there and the goals they want to see achieved along the way. Unfortunately, many people still think ’vision’ is the top leader’s job. In schools, the ‘vision task’ usually falls to the Headteacher and/or the governors or it comes in a glossy document from the local authority or the DfES. But visions based on authority are not sustainable. Making inclusive action plans using full participation and graphic facilitation Drawing on the planning tools MAPS and PATH (Pearpoint, Forest and O’Brien 1997) and other facilitation sources we use both process and graphic facilitation to enable the group to build their picture of what they would love to see happening within their organisation/community in the future and we encourage this to be a positive naming, not just a list of the things they want to avoid. Jack Pearpoint, Marsha Forest and John O’Brien developed these innovative approaches in North America and they are being used successfully in many parts of the UK. The planning can focus on an individual, group or organisation and provides a powerful problem solving opportunity, which is flexible and robust enough for many occasions. Tell the story, find the dream, touch the nightmare, and explore who you are, what are the gifts and strengths of the person or group, what are the needs of those present and what is the action plan for the future? Learning objectives Participants understand Person Centred Planning and its values and applications Participants have skills and confidence to facilitate PATH/MAP processes Participants learn graphic as well as process facilitation skills Strengthens practitioners inclusive practice Provides additional tools for those involved in inclusive work in schools and the community Further develop problem solving and planning skills Course Content The course answers the questions: Need to find new ways to bring Pathway Planning alive? Bored with annual reviews, transition plans and review meetings? Want to find a way of making meetings and planning feel more real and engaging? Need an approach, which engages a young person respectfully together with his or her family and friends? Want the ultimate visual record of the process of a meeting, which will help everyone, keep track? Want to problem solve and plan for the future of a small or large group, service or organisation up to the size of an LA? Inclusive Solutions offer an introductory day to person centred planning or a 3 – 10 session course which is practical as well as values based. Participants will receive direct individualised coaching and training. We will cover: The person being at the centre Family members and friends being full partners Planning reflecting the person’s capacities, what is important to the person and specifying the support they require to make a full contribution to their community Planning building a shared commitment to action that will uphold the person’s rights Planning leading to continual listening, learning and action and helping the person get what they want out of life. Essential Lifestyle Planning, PATH MAPS Personal Futures Planning.
It's no secret the world has been changing before our eyes !In order to confront the rapidly-evolving world around us, businesses need highly trained, adaptable and creative individuals to keep pace and stay innovative. BA (Hons) Business Management - 18 months course is designed to train you for the jobs of the future; you'll be taught the fundamentals of business management in the wider context of the contemporary business landscape, ensuring your skills will remain flexible and adaptable as the world changes. This BA (Hons.) Business Management - 18 Months programme aims to develop pro-active decision makers, managers and leaders for a variety of careers in business sectors in a global context. The course combines practical professional experience with creative approaches to enterprise and innovation that will develop your entrepreneurial spirit. Program Overview: BA (Hons) Business Management - 18 MonthsKey Highlights of BA (Hons) Business Management - 18 Months degree programme are: Program Duration: 18 Months (24 Months Option also available) Program Credits: 240 Designed for working Professionals 100% Online Global programme: Study anywhere, anytime on your laptop, phone or tablet. Study material: Comprehensive study materials and e-library support. No Written Exam. The Assessment is done via Submission of Assignment Online Lectures Timely Doubt Resolution Dedicated Student Success Manager Regular Networking Events with Industry Professionals LSBR Alumni Status Payment Plan: No Cost EMI Option

The Master of Business Administration (MBA) is a prestigious postgraduate qualification that is highly valued by leading employers. It can boost your salary, increase your professional reputation and expand your networking opportunities. If you're a graduate with some business experience and ambitions for a high-flying career, studying for an MBA could be just what you need to make the next step. Our MBA 12 months programme equips you to think logically, laterally and independently through 2 stage intensive, immersive, and challenging programme. With the advantage of studying on the job, anytime and anywhere, you get Cost Advantage and same degree which is given to full time students at the University Campus. The programme is not just an academic course. By exploring and examining real-life business problems to work on and solve, you enhance your own understanding of how a business works. We take a strategic perspective on business and management that helps you develop the skills to contribute to the major business decisions organisations have to make about their future. Program Overview: Master of Business Administration (MBA) - 12 Months Key Highlights of Master of Business Administration (MBA) - 12 Months qualification are: Fully Recognized and Globally Accepted Degree Program Duration: 12 Months (18 months / 24 months duration programme also available) Program Credits: 180 Designed for working Professionals Format: Online Student to faculty ratio of just 15:1 No Written Exam. The Assessment is done via Submission of Assignment and University Dissertation Project Same Degree which is given to Full Time students at the University Campus. Study material: Comprehensive study material and e-library support available at no additional cost. Tutor Assist available Dedicated Student Success Manager Timely Doubt Resolution Regular Networking Events with Industry Professionals Become eligible to gain direct entry into relevant Doctorate / PhD programme. LSBR Alumni Status No Cost EMI Option Top Skills You Will Learn MBA 12 months is widely seen as a passport to a successful career. It demonstrates the breadth and depth of your functional competence, strategic knowledge and problem-solving ability. Course Structure: MBA 12 MonthsThe MBA 12 months programme consists of 2 Stages.Stage 1: This stage is delivered by London School of Business and Research. The programme involves delivery through on-line Learning Management System (LMS). This stage leads to award of Level 7 Diploma in Strategic Management and Leadership. Credits earned at this stage - 120 credits (60 ECTS). Mandatory unitsStrategic Management (20 Credits)Strategic Leadership (20 Credits)Strategic Human Resource Management (20 Credits)Advanced Business Research Methods (20 Credits) Optional units(Choose any 2units to make 120 credits)Strategic Financial Management (20 Credits)Supply Chain and Operations Management (20 Credits)Entrepreneurship and Innovation (20 Credits)Globalisation and Corporate Governance (20 Credits)Strategic Change Management (20 Credits)Strategic Marketing (20 Credits) Successful completion of Stage 1 leads to Progression to Stage 2Stage 2: Delivered by the University / awarding body. On completion of the diploma programme you progress / Top up with Degree through a UK University for progression to the MBA degree. The stage 2 is delivered via distance learning by faculties from the University / awarding body. Credits earned at this stage - 60 credits (30 ECTS). Completion of Stage 2 leads to award of MBA Degree Dissertation Project Successful completion of Stage 2 leads to award of Degree by the university. Who is this course for? MBA in 12 Months programme is ideal for working professionals, successful managers, executives and professionals who want to take their career to a new level and Ambitious people who want to fast track their chosen career or start a new enterprise

Overview Internet is the main source of marketing today and Digital Marketing along with Social Media Marketing helps the business to stay alive in the digital world. Earlier companies use to spend a lot of money on traditional ways of marketing and still, they were not able to reach global clients. With the existence of Digital marketing, they are able to break the barriers to reaching global clients. With the use of Social Media Marketing and Digital Marketing organisations are able to reach every corner of the world and also it is very cost-effective. Digital Marketing encompasses various channels like Search Engine Optimisation, Social Media Marketing like usage of Facebook, Facebook marketing, Ad Words, and Email Campaigns and is list is longer. All these tools are used to cover global marketing. No matter where you are located in the world, your product can reach clients anywhere in the world. This course will help develop innovative social media strategies, and to boost brand awareness with rich content. With the effective analysis of campaign results, you'll be on track to exceed sales targets and advance your career with the latest social media marketing techniques.

Its no secret the world has been changing before our eyes ! In order to confront the rapidly-evolving world around us, businesses need highly trained, adaptable and creative individuals to keep pace and stay innovative. BA (Hons) Business Management course is designed to train you for the jobs of the future; you'll be taught the fundamentals of business management in the wider context of the contemporary business landscape, ensuring your skills will remain flexible and adaptable as the world changes. The programme aims to develop pro-active decision makers, managers and leaders for a variety of careers in business sectors in a global context. The course combines practical professional experience with creative approaches to enterprise and innovation that will develop your entrepreneurial spirit. Program OverviewKey Highlights Program Duration: 24 Months (18 Months Option also available) Program Credits: 120 Designed for working Professionals 100% Online Global programme: Study anywhere, anytime on your laptop, phone or tablet. Study material: Comprehensive study materials and e-library support. No Written Exam. The Assessment is done via Submission of Assignment Online Lectures Timely Doubt Resolution Dedicated Student Success Manager Regular Networking Events with Industry Professionals LSBR Alumni Status Payment Plan: No Cost EMI Option

Prospect Maturation
By EnergyEdge - Training for a Sustainable Energy Future
About this Training Course The prospect maturation process, from a lead to a drillable prospect, is at the heart of the exploration business. This 5 full day course will cover all aspects of the prospect maturation process: play understanding in the context of regional geological understanding, detailed prospect evaluation; realistic risk & volume assessment consistent with the play understanding and prospect details, and an introduction to exploration economics. Throughout the course, there is a strong focus on pragmatic (geo)logical approach for assessing those aspects that are input parameters for a meaningful assessment of prospect risks and volumes, with emphasis on a balanced integration of contributions from different sub-surface disciplines. Many examples from basins from around the world are used to illustrate how traps, reservoirs, seals and charge occur in different basin settings. Specifics topics that will be discussed include the following: The statistical fundamentals for risk and volume assessment will be presented, with practical exercises for understanding the results of a risk & volume assessment as they are displayed in expectation curves. The difference between risk and uncertainty. A full discussion of the essential requirements for a working petroleum system: Trap, reservoir, seal and charge. Examples of how traps, reservoirs, seals and charge work in different basin types around the globe and in Australian basins. Exercises and guidelines for estimating uncertainties for prospect parameters, including advice for deciding which distribution type to use, and how to constrain those distributions for meaningful uncertainty ranges (setting minimum most likely and maximum values). Particular emphasis will be given to estimating hydrocarbon column lengths with their associated uncertainties in undrilled prospects. Prospects and plays: The value of play maps and how these should be used for assessment of prospect risks and for ranking of prospects within a play. Calculating volume ranges for prospects. Calculating volumes for groups of prospects; how to add risked prospect volumes for a statistically correct representation of the volume promise of a portfolio of prospects. Geophysical evidence: Incorporating geophysical evidence (DHIs) consistently and realistically in a risk assessment. An understandable and geology-based workflow, consistent with Bayes theorem, will be presented. Exploration economics. Training Objectives What this course will cover in 5 days: This course describes the various aspects that need to be considered in the prospect maturation process, including: Play development in the context of a sound understanding of the regional geology Detailed prospect evaluation and understanding of the critical aspects of traps, reservoirs, seals and charge Examples from plays and prospects in different basin settings from around the globe Realistic and pragmatic risk and volume assessment, based on the geological understanding of plays and prospects An introduction to exploration economics Examples of plays, oil and gas fields and prospects from basins from around the world, including the Far East, will be given. Target Audience This course is designed primarily for Geoscientists in exploration who would like to improve their expertise of the prospect maturation process and risk and volume assessment. The course has proven to be of value for explorers in the early phase of their career, seasoned explorers and team leaders. It will also benefit staff from disciplines working closely with exploration staff including Prospect Portfolio Analysts, Petrophysicists, Geophysicists and Reservoir Engineers. Course Level Intermediate Training Methods At the end of the course, the participants will have a good understanding of the essentials for realistic risk and volume assessments of exploration prospects. The course should allow participants to produce well-considered and realistic assessments for prospects they may be working on, and to understand and constructively challenge risk and volume assessments of colleagues and/ or partners/ competitors. Each topic is introduced by a lecture, and learning is re-inforced by practical exercises and discussions. Hand-out materials in paper and/or electronic format will be provided. Time will be made available to discuss aspects of prospects that may be brought in by course participants. Trainer Your expert course leader has a PhD in Geology from the University of Utrecht. He worked for 31 years (1979 -2010) with Shell as an exploration geologist in a variety of functions across the globe. As Principle Technical Expert, he was responsible for ensuring that Risk & Volume assessments were carried out consistently and correctly in all of Shell's exploration units. In this capacity, he led and participated in countless prospect review sessions and developed and conducted a successful in-house course on Risks & Volume assessment. As manager of the Exploration Excellence Team, he performed in depth analysis of basins and plays and provided advice on exploration opportunities to senior management. Together with his team, he visited most of Shell's exploration offices, working hands-on with Shell's local exploration teams to generate new play and prospect ideas and to suggest evaluation techniques and technologies to apply. In 2010, he was appointed as extraordinary professor Regional and Petroleum Geology at the VU university of Amsterdam and in 2012 also at the University of Utrecht. He was visiting professor at the University of Malaya (Malaysia). Through his own consultancy, as of 2010, he provides advice on exploration activities to several companies and is regularly invited to carry out technical reviews. Activities cover all continents and include Portfolio Reviews, Prospect assessment, Play-based Exploration, and Geothermal activities. He conducts courses on several topics including Risk & Volume Assessment, Prospect Maturation, Basin Analysis, Play-based Exploration, Trap & Seal Analysis, Petroleum Geology for Non-geologists. Some of his recent publications include: De Jager, J. & van Winden, M. (2020): Play-Based Exploration of the petroleum potential of the Tremp-Graus, AÃnsa and eastern Jaca Sub-basins in the southern Pyrenees. Invited contribution for Digital Geology, EAGE special publication (eds: Grötsch, J. & Pöppelreiter, M.) De Jager, J. (2020). Concepts of Conventional Petroleum Systems. Invited contribution for Regional Geology and Tectonics Volume 1: Global Concepts, Techniques and Methodology (eds: Adam, J., Chiarelly, D. & Scarselli, N.) De Jager, J. (2021): Handbook Risk & Volume Assessment. Self-published De Jager, J., Van Ojik, K & Smit, R. (2023 - in preparation): Geological Development of The Netherlands. In: Geology of The Netherlands (eds: Ten Veen, J., Vis, G-J., De Jager, J. @ Wong, T.) POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information about post training coaching support and fees applicable for this. Accreditions And Affliations

Overview Financial Accounting and reporting play a very important role within the organization and its stakeholders. This course is designed to analyze the functions of financial reporting in communication and its effects on decision-making processes or managerial decisions. It will highlight the accounting and financial standards-setting process and its implication on the organization globally. Financial accounting and reporting discuss how accountants act as processors and purveyors of information for decision-making and the needs of those who use accounting information. It also looks at the role performed by accountants and notes the need to be aware of relevant regulatory and conceptual frameworks.

Overview Financial Analysis and reporting play a very important role within the organisation and its stakeholders. This course is designed to analyse the functions of financial reporting in communication and its effects on decision-making processes or managerial decisions. It will highlight the accounting and financial standards-setting process and its implication on the organisation globally. Financial Analysis and reporting discuss how accountants act as processors and purveyors of information for decision-making and the needs of those who use accounting information. It also looks at the role performed by accountants and notes the need to be aware of relevant regulatory and conceptual frameworks.

Overview Corporate frauds have the inherent power to bring large organizations to their knees, cause huge monetary loss, prompt lawsuits followed by significant legal expenses, lead to the imprisonment of employees and deteriorate confidence in the market, governments, and institutions. In response, corporations and governments across the globe have stepped up their effort to inspect, prevent and penalize fraudulent practices; resulting in a greater emphasis on the domains of forensic auditing and accounting in the current economy. This training course will empower you to recognize the root causes of fraud and white-collar crime in the current economy, understand the categories of fraud, equip you with methodologies of fraud detection and prevention, and heighten your ability to detect potential fraudulent situations. In addition to the fundamentals of fraud investigation and detection in a digital environment; profit-loss evaluation, analysis of accounting books, legal concepts, and quantification of financial damages are also examined in this course

