- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
143 Courses delivered Online
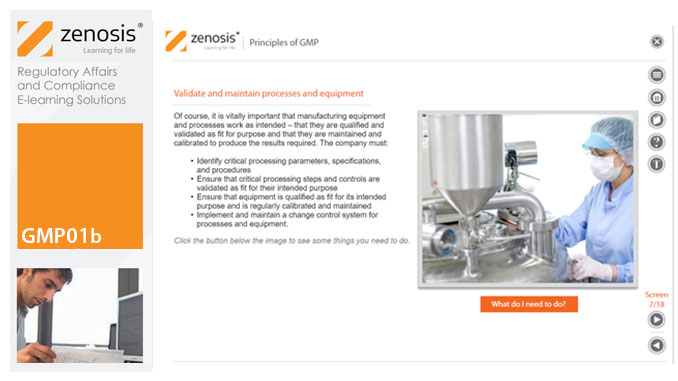
GMP01b - Principles of GMP
By Zenosis
In this short course we present an overview of the main principles of GMP, and we outline some things that manufacturing personnel need to do to comply with requirements. We identify the principal goals of GMP as: prevention of contamination; prevention of mix-ups; scrupulous documentation; validation and maintenance of processes and equipment; quality assurance by an independent unit; and training. We place GMP in the context of a company’s quality management system.
CT04b - Clinical protocol design
By Zenosis
Clinical trial protocols are an essential part of clinical trial design. Protocol documents are critical to conducting safe and cost-effective investigations. Protocol documents are large and complex, containing comprehensive information relating to purpose, design and conduct of a clinical trial. Aspects of a protocol include patient eligibility criteria, and treatment specifications. This short course provides an overview of clinical trial protocols. Opportunities to improve a clinical trial protocol for regulatory approval are also discussed.
Video Editing for Beginners Short Course Mini Bundle
By Compete High
The Video Editing for Beginners Short Course Mini Bundle is your entry into the world of digital visuals—minus the headache of over-complicated tech talk. You’ll explore Adobe Premiere Pro, video planning, drawing fundamentals, basic animation, and time management (because editing takes longer than you'd expect). If you’ve ever tried to cut a 30-minute video into something that doesn’t bore people by minute two, you already know the value of knowing your tools. This course keeps it structured, simple, and creative—perfect for people ready to edit without overthinking the timeline. Learning Outcomes: Edit and cut videos using Adobe Premiere Pro software. Understand the basics of animation and motion graphics. Plan and organise ideas for better video development flow. Apply drawing concepts for layout or visual storytelling. Improve time management when working on creative projects. Use software tools effectively for beginner video editing. Who Is This Course For: Beginners exploring video editing for creative or casual use. Content creators looking to edit their own footage confidently. Social media users making engaging videos and reels. Freelancers offering editing alongside other creative services. Bloggers and vloggers wanting more polished video content. Students learning creative tools outside of formal environments. Professionals creating videos for business or team updates. Anyone tired of using ten apps to crop one clip. Career Path: Junior Video Editor – £26,000/year Content Creator – £27,000/year Social Media Video Assistant – £25,000/year Animation Intern – £23,000/year Marketing Assistant (Video Focus) – £28,000/year Freelance Editor (Entry-Level) – £24,000/year

CT04c - Clinical trial preparation
By Zenosis
The demands on quality from clinical trials are increasing. Quantitative aspects of clinical trials, such as the mass of study data to be collected, the multiple investigational sites, and the need to meet predetermined timelines, often supersede qualitative features. Therefore, addressing basic requirements for quality management is essential when preparing a clinical trial. This short course describes the core elements required for the establishment of a clinical trial and provides an overview of the role of the sponsor in supporting and improving trial quality.
CT04g - Data capture and management in clinical trials
By Zenosis
Capture and management of clinical trial data is a challenge. The industry is under pressure to obtain and analyse such data more quickly, while maintaining data integrity, so that products can be brought to market sooner. Effective planning and adequate resources can ensure clinical trials yield high quality data within strict timelines and budget requirements, at the same time satisfying regulatory standards. This short course describes the purpose of data capture and explores efficiencies in data management as part of the evolving regulatory landscape.
CT03a - ICH, harmonisation, and principles of Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data in applications for marketing approval. The International Council for Harmonisation's guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice. In this short course we describe the ICH’s role in the harmonisation of regulations, introduce its guideline E6, and set out the principles of GCP.
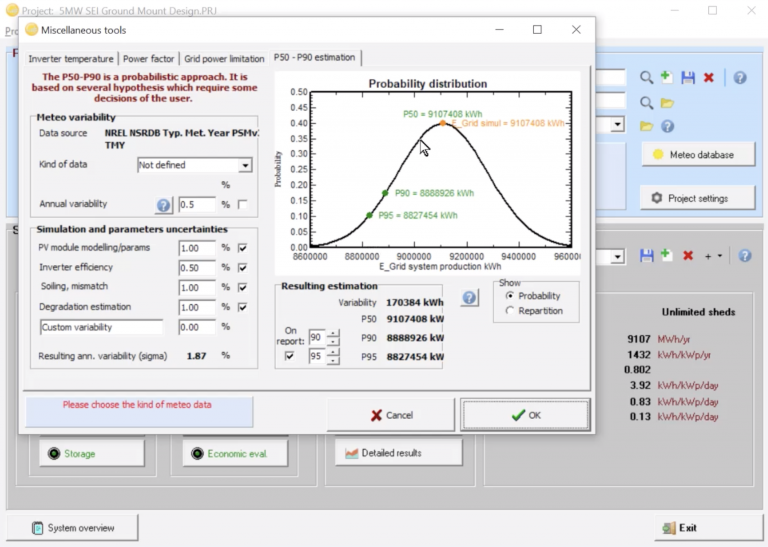
CE524: PVsyst for PV System Production Modeling
By Solar Energy International (SEI)
This short course is targeted towards beginning users, and will show you in detail how to get started creating accurate production estimates for any size PV system, from residential to large-scale. Learn how to find and import the correct meteorological data, create system variants for any size system, and accurately define the orientation, shading scene, and detailed system losses. By the end of this course you will be confidently simulating production and printing reports to share.
CT04a - Clinical trials in drug development
By Zenosis
New drug development requires major investment in capital, human resources and technical expertise. Strict adherence to regulations on testing and manufacturing standards is also required before a new drug can be marketed. One of the greatest challenges in conducting clinical trials is that of efficiency. As trials become more comprehensive, involving large numbers of participants globally, their duration is prolonged and costs increase. The longer trials last, the shorter is the patent life remaining after market approval and the longer patients must wait for the new product. This short course covers the key components of clinical trials and how these requirements interact with the drug development cycle.

CT04d - Clinical trial endpoints
By Zenosis
In clinical trials, endpoints are measurements to evaluate the results of a new treatment, at an individual patient level. The study data can be extrapolated to patient populations on the basis of clinical similarities to patients participating in the trial. When clinical trial data have been obtained, focus is on the trial endpoints; more specifically, the focus is on whether the trial met or failed the primary endpoint specified before the trial started. The purpose and various types of endpoints are discussed in this short course.
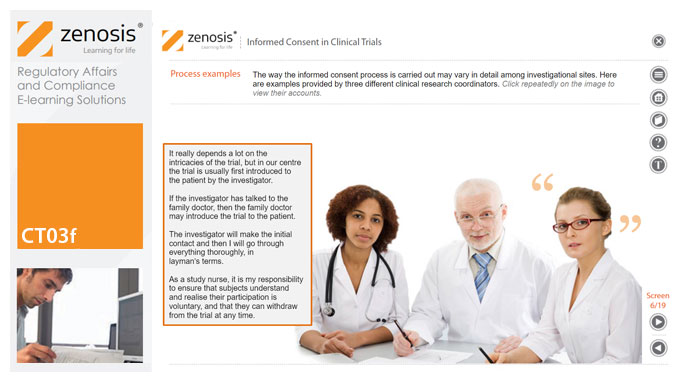
CT03f - Informed consent in clinical trials
By Zenosis
Informed consent in clinical research is an ethical and regulatory requirement. A research subject must enter a study voluntarily, be informed about risks and benefits, and understand the difference between investigation and treatment. Subjects must not be coerced into enrolment, nor must they be enticed by exaggerated claims of benefit. Before they can enrol, all potential subjects must agree, in writing, to participate. In addition to ethical and regulatory imperatives, the potential for litigation by subjects further highlights the importance of rigorous adherence to informed consent principles. In this short course we set out the principles and requirements and provide examples of practical issues confronting healthcare professionals and subjects.