- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
294 Courses delivered Online
ESS01: Essentials of EU and US Regulatory Affairs for Human Medicinal Products
By Zenosis
This foundation-level module is the ideal introduction for new entrants to the field of pharmaceutical regulatory affairs and compliance. It describes the principal requirements that must be satisfied to gain and maintain approval to market medicinal products in the USA and Europe. The legal framework and the roles of major players in regulation are presented. The life-cycle of a drug is outlined. The various procedures available for assessment and approval of products are described and their requirements outlined. Obligations to be fulfilled after marketing approval are discussed.

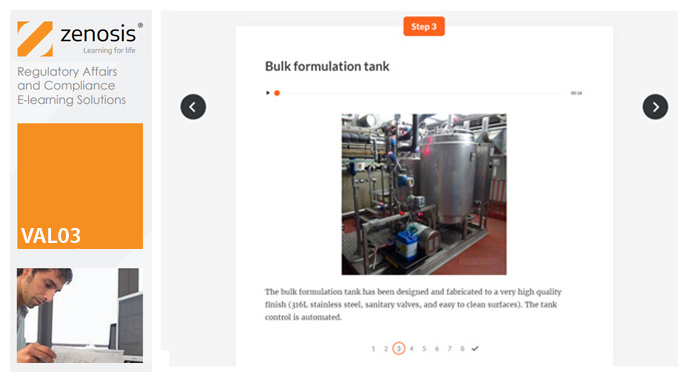
VAL03: Commissioning and Installation Qualification
By Zenosis
Before equipment can be used routinely in production, it must first be commissioned and, if necessary, undergo Installation Qualification (IQ). This module describes commissioning and IQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
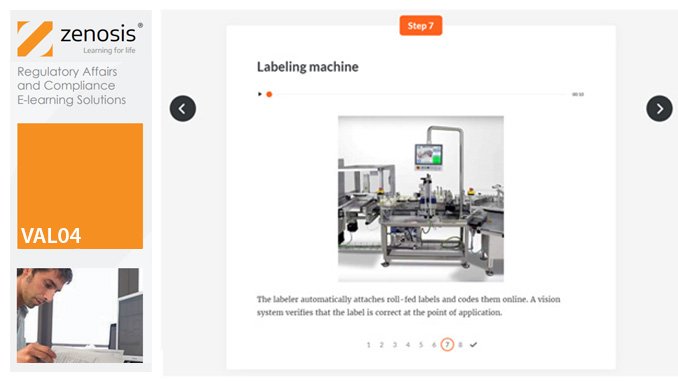
VAL04: Operational and Performance Qualification
By Zenosis
Having undergone Installation Qualification, before equipment can be used routinely in production, it needs to undergo Operational Qualification (OQ) and Performance Qualification (PQ). This module describes OQ and PQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
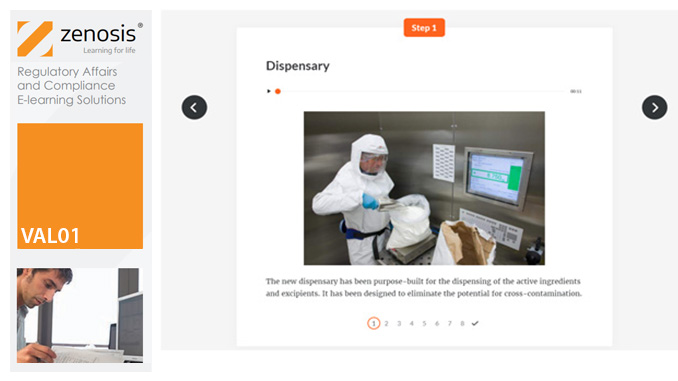
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
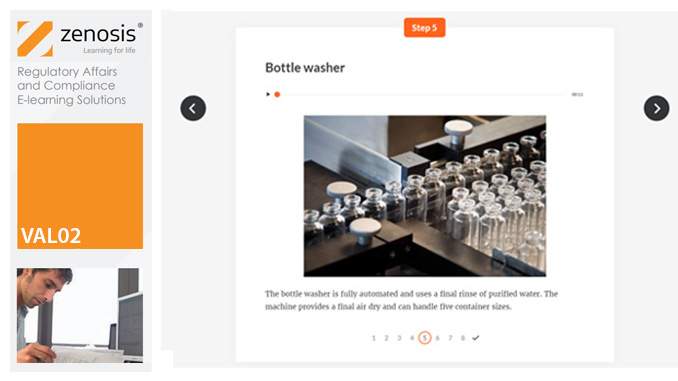
VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
Pharmacy Skills, Medicine Management & Medical Law
By Imperial Academy
Pharmacy Skills, Medicine Management & Medical Law

Laboratory Technician (Microbiology, Infection Prevention and Control)
By Imperial Academy
3 QLS Endorsed Diploma | QLS Hard Copy Certificate Included | 10 CPD Courses | Lifetime Access | 24/7 Tutor Support

CT07: An Introduction to Clinical Trials and Drug Development
By Zenosis
This module provides an understanding of how clinical trials fit into the drug development process. It outlines the key historical events leading to the development of controlled clinical trials. It specifies the purpose of trials, outlines their features, and identifies codes and regulations that apply to them. Finally, it describes the environment of cost control in which the modern pharmaceutical industry operates.
Biotechnology, Genetics & Biochemistry (QLS)
By Imperial Academy
3 QLS Endorsed Diploma | QLS Hard Copy Certificate Included | 10 CPD Courses | Lifetime Access | 24/7 Tutor Support

