- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Start learning Clinical Observation Training: Health Safety and Medicine that will give you enough knowledge and skills to build your dream career. About this course This Clinical Observation Training: Health Safety and Medicine helps to grow your skills faster through the power of relevant content and world-class tutors. In this industry-leading bite-sized course, you will learn up-to-date knowledge in the relevant field within a few hours and get certified immediately. The modules of this course are very easy to understand and all of the topics are split into different sections. You will easily grasp and use the knowledge gained from this course in your career and go one step ahead of your competitors. The course is designed to improve your employability and provide you with the tools you need to succeed. Enrol today and start learning your essential skills. Why choose this course Earn a digital Certificate upon successful completion. Accessible, informative modules taught by expert instructors Study in your own time, at your own pace, through your computer tablet or mobile device Get 24/7 help or advice from our email and live chat teams Get full tutor support on weekdays (Monday to Friday) Course Design The Clinical Observation Training: Health Safety and Medicine is delivered through our online learning platform, accessible through any internet-connected device. There are no formal deadlines or teaching schedules, meaning you are free to study the course at your own pace. You are taught through a combination of Video lessons Online study materials Who Is This Course For:â This Clinical Observation Training: Health Safety and Medicine is ideal for those who want to be skilled in this field or who wish to learn a new skill to build their dream career. If you want to gain extensive knowledge, potential experience, and be an expert in the related field then this is a great course for you to grow your career. Requirements This course is for anyone who would like to learn Clinical Observation Training: Health Safety and Medicine related skills to aid his/her career path. No formal entry prerequisites are required Certification Upon successful completion of the course, you will be able to obtain your course completion e-certificate. Print copy by post is also available at an additional cost of £9.99 and PDF Certificate at £4.99. Course Content Module 01: An Overview of Clinical Observation and Clinical Career Module 02: Observation of Clinical Management Module 03: Observation of technical clinical method Module 04: Observation of Medicine and Pharmacies Module 05: Hygiene and Health Safety Course Content Clinical Observation: Health Safety and Medicine Module 01: An Overview of Clinical Observation and Clinical Career 00:08:00 Module 02: Observation of Clinical Management 00:13:00 Module 03: Observation of technical clinical method 00:20:00 Module 04: Observation of Medicine and Pharmacies 00:10:00 Module 05: Hygiene and Health Safety 00:17:00 Order your Certificates & Transcripts Order your Certificates & Transcripts 00:00:00 Frequently Asked Questions Are there any prerequisites for taking the course? There are no specific prerequisites for this course, nor are there any formal entry requirements. All you need is an internet connection, a good understanding of English and a passion for learning for this course. Can I access the course at any time, or is there a set schedule? You have the flexibility to access the course at any time that suits your schedule. Our courses are self-paced, allowing you to study at your own pace and convenience. How long will I have access to the course? For this course, you will have access to the course materials for 1 year only. This means you can review the content as often as you like within the year, even after you've completed the course. However, if you buy Lifetime Access for the course, you will be able to access the course for a lifetime. Is there a certificate of completion provided after completing the course? Yes, upon successfully completing the course, you will receive a certificate of completion. This certificate can be a valuable addition to your professional portfolio and can be shared on your various social networks. Can I switch courses or get a refund if I'm not satisfied with the course? We want you to have a positive learning experience. If you're not satisfied with the course, you can request a course transfer or refund within 14 days of the initial purchase. How do I track my progress in the course? Our platform provides tracking tools and progress indicators for each course. You can monitor your progress, completed lessons, and assessments through your learner dashboard for the course. What if I have technical issues or difficulties with the course? If you encounter technical issues or content-related difficulties with the course, our support team is available to assist you. You can reach out to them for prompt resolution.
CT04a - Clinical trials in drug development
By Zenosis
New drug development requires major investment in capital, human resources and technical expertise. Strict adherence to regulations on testing and manufacturing standards is also required before a new drug can be marketed. One of the greatest challenges in conducting clinical trials is that of efficiency. As trials become more comprehensive, involving large numbers of participants globally, their duration is prolonged and costs increase. The longer trials last, the shorter is the patent life remaining after market approval and the longer patients must wait for the new product. This short course covers the key components of clinical trials and how these requirements interact with the drug development cycle.

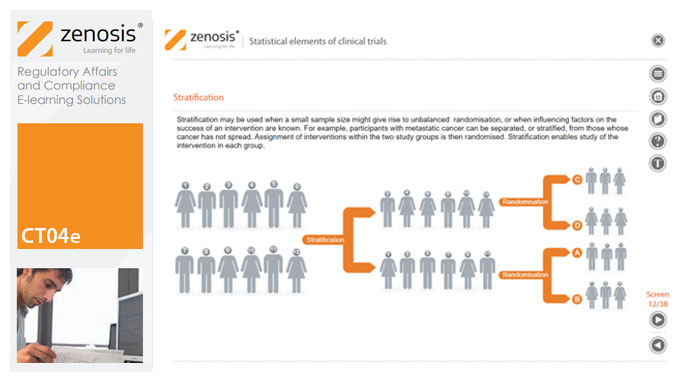
CT04e - Statistical elements of clinical trials
By Zenosis
Analytical statistical elements are essential concepts in the design of clinical trials. This analysis helps us to understand whether a conclusion from a study of a sample of the target population applies generally to that population as a whole. In particular, it helps us to answer the question: Did the treatment effect in the given study occur just by chance? The statistical elements of a well-controlled study minimise the chances of drawing the wrong conclusions, by providing clear thresholds for such errors. The basic statistical elements of a clinical trial include eligibility criteria, randomisation, sample size, power, and blinding, and these are discussed in this short course.
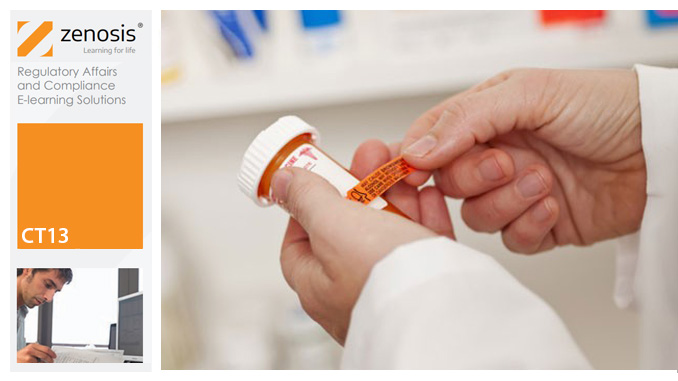
CT13: Safety Reporting in Clinical Trials
By Zenosis
This course explains the regulatory requirements for the reporting of adverse events and suspected adverse reactions in clinical trials. It describes how investigators should report to sponsors, and how sponsors should report to regulatory authorities and other stakeholders in the safety of investigational products. It explains how events are characterized as serious or non-serious, expected or unexpected, and it distinguishes the requirements for each category. It describes controlled vocabularies used for coding of events in reports.
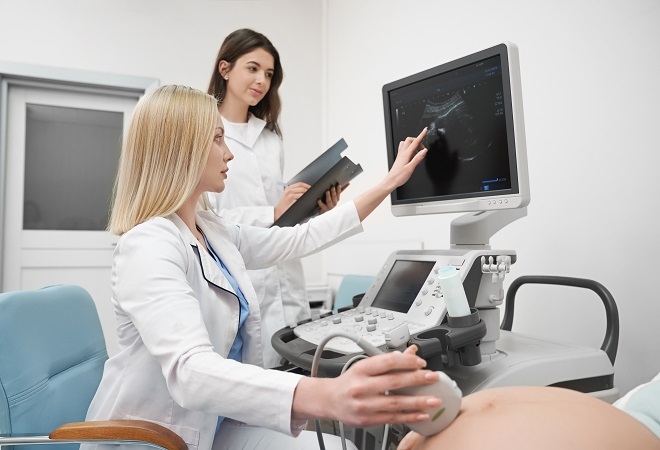
Laboratory Technician Level 7 - QLS Endorsed
By Kingston Open College
QLS Endorsed + CPD QS Accredited - Dual Certification | Instant Access | 24/7 Tutor Support | All-Inclusive Cost

Elevate your career in healthcare with our comprehensive Clinical Chemistry and Medical Laboratory Technician course. Gain expertise in laboratory techniques, clinical chemistry analysis, immunology, and more. Ensure precision with quality assurance, laboratory safety, and cutting-edge technologies. Join us for hands-on training and advance your skills in a dynamic and vital field. Enroll now for a rewarding journey in medical laboratory services.

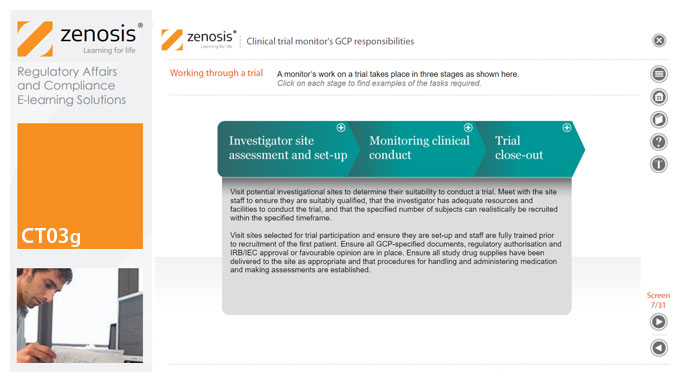
CT03g - Clinical trial monitor’s GCP responsibilities
By Zenosis
A clinical trial monitor acts on behalf of the sponsor to support investigational site personnel, verify the accuracy of data recorded, and ensure that the trial is conducted in compliance with the protocol, GCP and other study specific requirements. He or she acts as the ‘eyes and ears’ of the sponsor at the investigational site and provides the main channel of communication between sponsor and investigator. This short course explores the responsibilities of the monitor and provides insight into key challenges. We discuss assessment of investigators and investigational sites, education and trial initiation, monitoring of clinical conduct, including CRF review and source document verification, and trial close-out. We discuss noncompliance and how to deal with it.
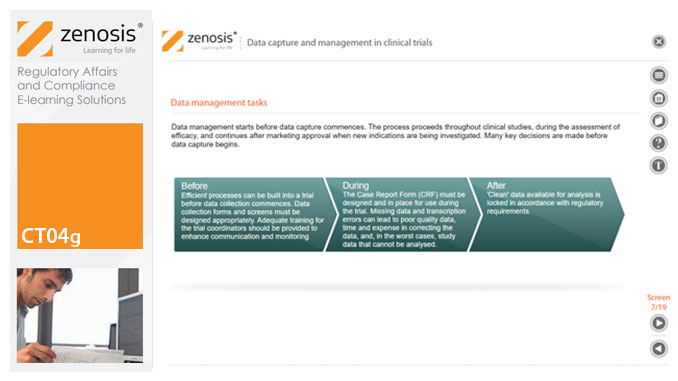
CT04g - Data capture and management in clinical trials
By Zenosis
Capture and management of clinical trial data is a challenge. The industry is under pressure to obtain and analyse such data more quickly, while maintaining data integrity, so that products can be brought to market sooner. Effective planning and adequate resources can ensure clinical trials yield high quality data within strict timelines and budget requirements, at the same time satisfying regulatory standards. This short course describes the purpose of data capture and explores efficiencies in data management as part of the evolving regulatory landscape.
CT03d - Clinical trial sponsor’s GCP responsibilities
By Zenosis
The sponsor of a clinical trial takes responsibility for its initiation, management, and/or financing. A sponsor may transfer any or all of the sponsor’s trial-related duties and functions to a contract research organisation, but the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor. Duties and functions discussed in this short course include trial design, selection of investigators, QA and QC, data handling and record keeping, finance and compensation, regulatory submissions, management of investigational product(s), safety reporting, monitoring, audit, dealing with noncompliance, and clinical trial reports. ICH guideline E6 (revision 2) encourages sponsors to adopt a risk-based approach to managing the quality of trials. We discuss this approach in general, and aspects such as risk-based monitoring in particular.
Overview Perks of being a medical coder are endless. Medical coders can earn handsome salaries and have great opportunities for advancement. These professionals are essential to all areas of healthcare. Kickstart your career in medical coding by taking this Medical Coding Certification Course. Transforming healthcare diagnosis, procedures, medical services, and equipment into applicable medical codes is known as medical coding. Medical coders extract patient data from medical charts created by physicians regularly. Through this Medical Coding Certification course, you'll explore the fundamentals of medical billing and coding. The program will explain the medical coding process in great detail. Moreover, it will look into the aspects of medical coding terminology and CPT codes in medical billing. Furthermore, it will take you through the medical billing and insurance claims process and give you insights into medical office technology. Learning Outcomes Learn the steps in the medical coding process Discover the different methods of medical coding Find a thorough overview of CPT codes in medical billing Enrich your knowledge of the Medical Billing and Insurance Claims Process Familiarise yourself with the common billing and coding mistakes and how to avoid them Who is the Course for? This Medical Coding Certification course is suitable for anyone interested in gaining exceptional clinical coding skills. The training will open doors to various career opportunities in the healthcare industry. Entry Requirement This course is available to all learners, of all academic backgrounds. Learners should be aged 16 or over to undertake the qualification. Good understanding of English language, numeracy, and ICT are required to attend this course. Why Choose Us? Affordable, well-structured and high-quality e-learning study materials Engaging tutorial videos, materials from the industry-leading experts Opportunity to study in a user-friendly, advanced online learning platform Efficient exam systems for the assessment and instant result UK & internationally recognised accredited qualification Access the course content on mobile, tablet, or desktop from anywhere, anytime Excellent career advancement opportunities 24/7 student support via email. Assessment At the end of the course, you will be required to sit for an online multiple-choice test. Your test will be assessed automatically and immediately so that you will instantly know whether you have been successful. Certificate of Achievement After completing this course successfully, you will be able to obtain an Accredited Certificate of Achievement. Certificates & Transcripts can be obtained either in Hardcopy at £14.99 or in PDF format at £11.99. Career Path Medical Coding Certification Course provides useful skills to possess, and would be beneficial for the following professionals: Clinical coder Medical administrator Medical Coding Certification Module 01: Anatomy and Physiology 01:00:00 Module 02: The Chemical Level of Organisation 00:20:00 Module 03: Cell 00:50:00 Module 04: Tissues and Membranes 00:35:00 Module 05: Skeletal System 01:00:00 Module 06: Muscular System 00:30:00 Module 07: Cardiovascular System 00:35:00 Module 08: Digestive System 00:15:00 Module 09: The Endocrine System 00:50:00 Module 10: The Nervous System 01:00:00 Module 11: The Respiratory System 00:30:00 Module 12: The Immune System 00:40:00 Module 13: The Urinary System 00:20:00 Module 14 The Reproductive System 01:30:00 Module 15: The Integumentary System 00:35:00 Module 16:The Senses 00:50:00 Module 17: Medical Terminology 00:35:00 Module 18: Medical Coding: CPT 00:35:00 Module 19: ICD 10 Codes 00:30:00 Module 20: Medical Office Administration 00:30:00 Module 21: Medical Billing and Insurance Claims Process 00:20:00 Module 22: Medical Office Technology 00:15:00 Module 23: Ten Common Billing and Coding Mistakes and Avoiding Them 00:10:00 Mock Exam Mock Exam - Medical Coding Certification 00:20:00 Final Exam Final Exam - Medical Coding Certification 00:20:00 Order Your Certificates and Transcripts Order Your Certificates and Transcripts 00:00:00
