- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
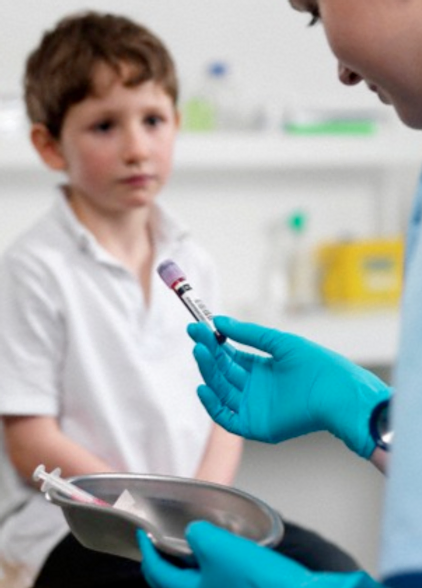
Specialised techniques and skills associated with neonatal and paediatric blood draws Nationally Recognised Qualification OCN Accredited - Level 3 (advanced) CPD Accredited - The CPD Certification Service Follow-on from Introduction to Phlebotomy Course Complements our Advanced Phlebotomy Course (Level 4 - FDSc level) Expand your horizons and add new skills Covers neonates, infants and child draws Legal framework and consenting Download a certificate on completion of your online course FOLLOWS ON FROM INTRODUCTION TO PHLEBOTOMY COURSE BUT ALSO OPEN TO ALL APPLICANTS
Assisting patients at the end of their life's journey ... Nationally Recognised Qualifications Accredited OCN Credit4Learning - Level 3 Accredited CPD (The CPD Certification Service) Expand your horizons to include this specialised area of caring Comprehensively covers end of life and terminal patient care skills Includes support and reference material to download and keep No previous experience or qualification needed Download a certificate on completion of your online course
Want to get started in healthcare? A beginner's course and first step onto the healthcare career ladder Nationally Recognised Qualification Accredited with Open College Network OCN Credit4Learning Level Three Certificate (advanced) Ideal for healthcare assistants / carer positions Essential home practical caring skills Comprehensively covers fundamental care skills Includes support and reference material to keep Easy to follow and fun to learn Ideal for freelancer carers No previous experience or qualification needed Download a certificate on completion of your online course OPEN TO ALL APPLICANTS

Understanding specific words and terms used in the healthcare sector is an absolute must if you are looking to progress in this profession. Once you have a basic understanding of how medical words are constructed they become easy to understand and use are internationally used by nurses, doctors, allied healthcare professionals, dentists and many other medical specialities.

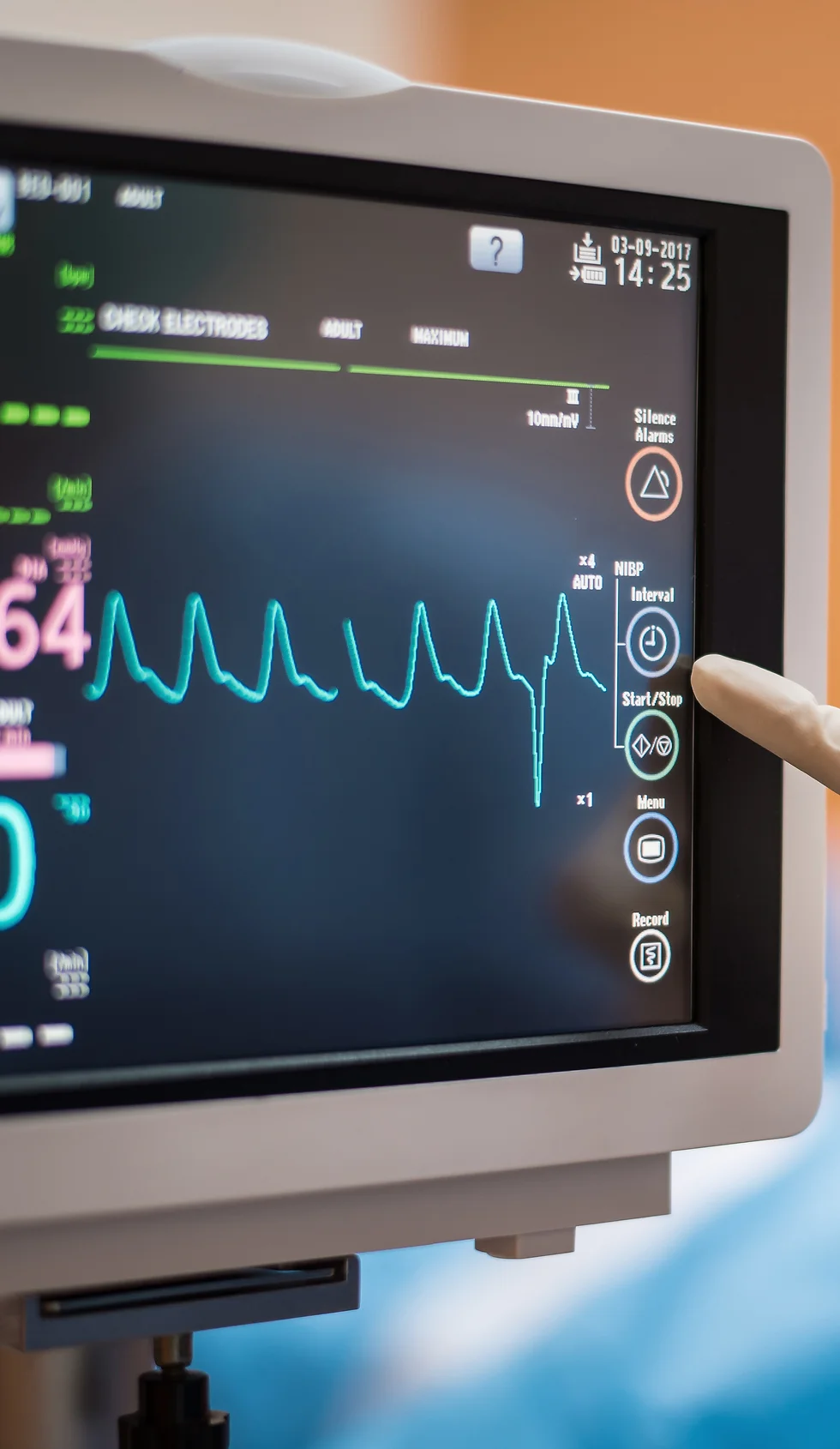
Learn how to perform and read an ECG ... Nationally Recognised Qualification OCN Accredited - Level 3 (advanced level) CPD Accredited - The CPD Certification Service Introduces you to the fundamentals of setting up and operating an ECG machine Includes patient preparation Produce a valid (error free) ECG Learn and understand ECG traces Recognise recordings that require urgent attention Basic understanding of English language required OPEN TO ALL APPLICANTS VIRTUAL CLASSROOM OPTION INCLUDES COMPREHENSIVE PRACTISE@HOME ECG TRAINING KIT Final interpretation of all ECG recordings is the responsibility of a medical professional.
THIS COURSE PACKAGE INCLUDES: 1: INTRODUCTION TO ECG COURSE - RECORDING & BASIC INTERPRETATION (GPT009) 2: ADVANCED ECG COURSE - INTERPRETATION & ANALYSIS (GPT010) Learn how to set up and record a basic ECG trace, followed by advanced analysis and interpretation FAST-TRACK YOUR ECG TRAINING WITH OUR BEGINNER TO ADVANCED TRAINING PACKAGE 20% off - Multi-Course Discount Cover all stages from Level 1 through to Level 4 (FDSc) Cover your theory training online Practical training in Classroom or Virtual Classroom Comprehensive Practise@Home training kits for VC Awards 2 accredited qualifications Dual Accreditations are awarded for all courses (Open College Network and CPD) Covers all steps required to competently set up and perform an ECG trace. Practical sessions include electrode placement on mannequin, running traces and identifying anomalies. Learn beginner to advanced skills and interpretation. Basic understanding of English language required. OPEN TO ALL APPLICANTS About these courses 1: INTRODUCTION TO ECG COURSE - RECORDING AND BASIC INTERPRETATION (GPT009) PART 1 - Theory Allow approx. 5-6 hours PART 2 - Practical Training Attend a classroom location or join us in our virtual classroom * - 3-4 hours ACCREDITED LEVEL 3 QUALIFICATION * Virtual Classroom option includes a free comprehensive Practise@Home ECG training kit. 2: ADVANCED ECG COURSE - INTERPRETATION AND ANALYSIS (GPT010) E-LEARNING - Theory Allow approx. 6-8 hours ACCREDITED LEVEL 4 QUALIFICATION OPTIONAL: GETTING STARTED IN ECG (GPT002) A free starter ECG Course (unassessed) developed to help you understand the basics of ECG recording: 3 modules in total with no Questions! If you are already familiar with ECGs then you may prefer to save time and opt out of this mini-course at booking stage. This "mini-course" is available at no charge. Learning Outcomes GPT009: Understanding different ECG equipment types ECG equipment - set-up and calibration Includes professionalism, consent, IPC and legal requirement Patient preparation How to correctly apply electrodes to limbs and chest Identify artifacts (equipment and patients Identify and recognise routine traces Identify and recognise non-routine traces Identify traces requiring urgent attention Labelling and reporting GPT010: Understand the acceptable variations within the normal ECG of healthy adults. Recognise the expected patterns of an ECG from a healthy child from birth onwards and identify abnormalities. Interpret abnormal ECG patterns in adults. Diagnose arrhythmias as an underlying cause of palpitations and syncope. Exploring sinus rhythm, extrasystoles, paroxysmal tachycardia and the importance of a physical examination. Identifying syncopal episodes attributable to cardiovascular disease as opposed to arrhythmias. Recognise ECG markers for tachycardias, bradycardias, pre-excitation syndromes, bi-fascicular block, and first-degree block with bundle branch block. Differentiate between supraventricular and ventricular extrasystoles and be able to diagnose broad complex tachycardias, ventricular flutter and fibrillation, sick sinus syndrome, and Stokes-Adams attacks. Recognise and identify symptoms associated with the causes of acute or chronic chest pain in patients who present with myocardial infarction (heart attack), pulmonary embolism, significant central pulmonary embolism, pericarditis, aortic dissection, oesophageal rupture, spinal disorders, vertebral collapse, posterior infarction, and angina. Recognise symptoms indicative of conditions such as pulmonary oedema, chest diseases, and pulmonary congestion. After the course GPT009: Safely and competently set up an ECG machine Introduce patients to the ECG test, adhering to compliancy requirements before and after testing Perform an ECG test to national guidelines Understand basic traces and their correlation to cardiac issues Recognise normal and erroneous recordings Recognise recordings that require urgent medical follow-up Complete the recording and label (or record digital copies) as per guidelines GPT010: Appreciate normal and abnormal ECG variations in the context of varying pathologies. Be able to determine whether an arrhythmia has an underlying cause that requires medical intervention. Interpret ECGs as a function of the patient's ongoing cardiac management. Understand and apply the Burce Protocol exercise test in relevant clinical situations. Know how to clinically respond to a patient with chest pain including further investigations required, pain relief, history and examination and echocardiogram. Understand and apply the fundamental principles of arrhythmia management. Understand the primary causes of heart disease and the diagnostic process. Appreciate the importance of the ECG as a diagnostic tool alongside the patient’s history and clinical presentation and recognising its limitations. Course Package Components: PACKAGE - Beginner to Advanced ECG - Virtual Classroom - INTRO - Part 1 online Part 2 Virtual Classroom (AM) + ADVANCED - E-learning