- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

Ofqual Regulated Level 3 Teaching Assistant Award & EYFS Teaching Diploma
By Kingston Open College
Premium Bundle of all Time | Ofqual Regulation + NCFE Awards + CPD Accreditation | Assessment & Tutor Support Included

Microsoft Word Intermediate - In-company (now with live online classes)
By Microsoft Office Training
Course Objectives At the end of this course you will be able to: Customise tables and charts Customise formatting with styles and themes Modify pictures in a document Create customised graphic elements Insert content using Quick Parts Control text flow Use templates to automate document creation Perform mail merges Use macros to automate common tasks '1 year email support service Take a look at the consistent excellent feedback from our corporate clients visiting our site ms-officetraining co uk Customer Feedback Excelent enviroment for training. Tahnk you. Jenny Price - ACER ' With more than 20 years experience, we deliver courses on all levels of the Desktop version of Microsoft Office and Office 365; ranging from Beginner, Intermediate, Advanced to the VBA level. Our trainers are Microsoft certified professionals with a proven track record with several years experience in delivering public, one to one, tailored and bespoke courses. Our competitive rates start from £550.00 per day of training Tailored training courses: You can choose to run the course exactly as they are outlined by us or we can customise it so that it meets your specific needs. A tailored or bespoke course will follow the standard outline but may be adapted to your specific organisational needs. Working with Tables and Charts Sort Table Data Control Cell Layout Perform Calculations in a Table Create a Chart Customising Formats Using Styles and Themes Create and Modify Text Styles Create Custom List or Table Styles Apply Document Themes Using Images in a Document Resize an Image Adjust Image Appearance Integrate Pictures and Text Insert and Format Screenshots Use the Snipping tool Creating Custom Graphic Elements Create Text Boxes and Pull Quotes Draw Shapes Add WordArt and Other Text Effects Create Complex Illustrations with SmartArt Inserting Content Using Quick Parts Insert Building Blocks Create and Modify Building Blocks Insert Fields Using Quick Parts Controlling Text Flow Control Paragraph Flow Insert Section Breaks Insert Columns Link Text Boxes to Control Text Flow Using Templates Create a Document Using a Template Create a Template Using Mail Merge The Mail Merge Features Merge Envelopes and Labels Create a Data Source Using Word Using Macros Automate Tasks Using Macros Create a Macro Who is this course for? Who is this course for? This course is designed for users who to create or modify complex business documents as well as customised Word efficiency tools Requirements Requirements Preferably, delegates should have attended the Word Introduction course. Career path Career path Microsoft Office know-how can instantly increase your job prospects as well as your salary. 80 percent of job openings require spreadsheet and word-processing software skills Certificates Certificates Certificate of completion Digital certificate - Included

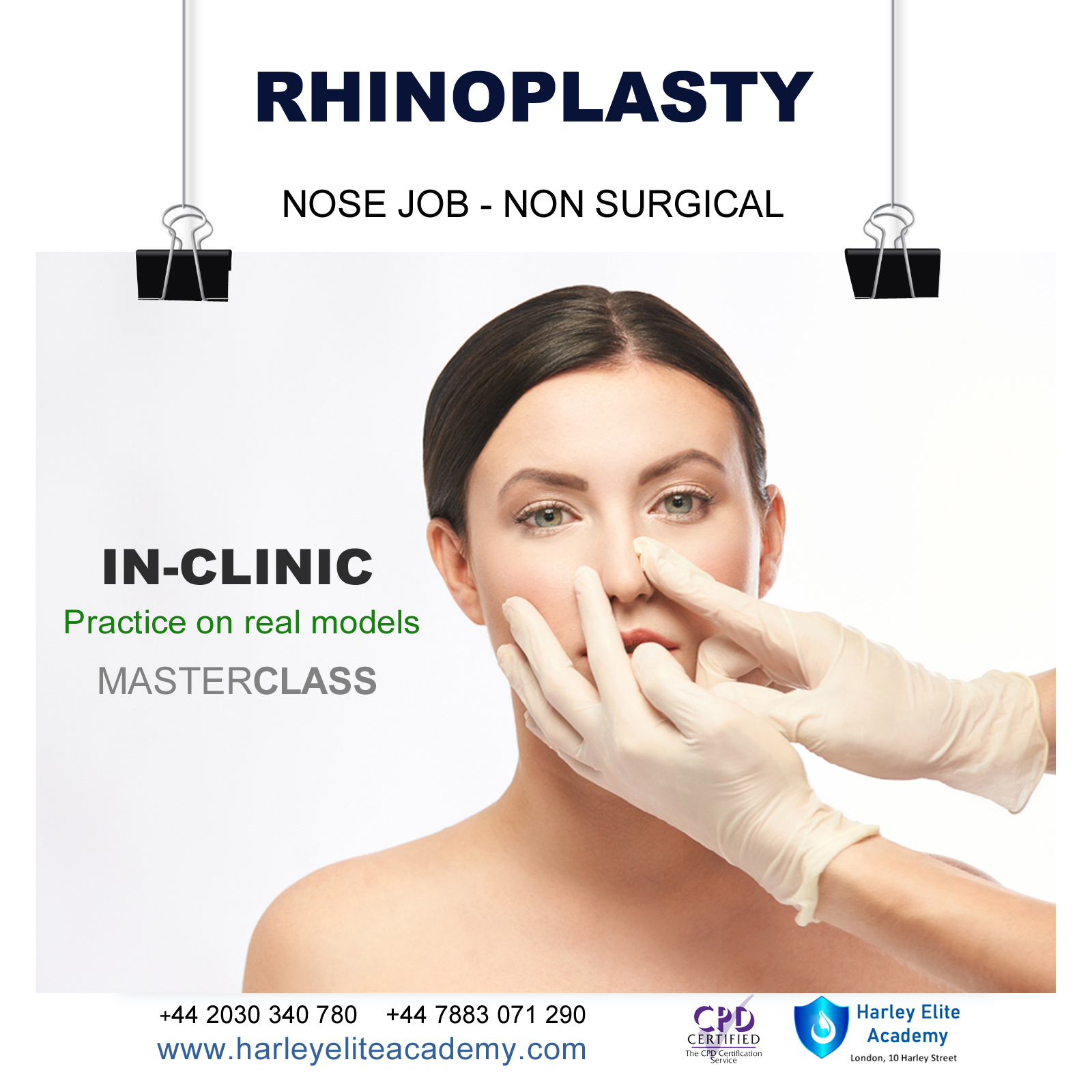
RHINOPLASTY NOSE JOB COURSE
By Harley Elite Academy (HeLa)
MASTERCLASS 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! On this course, we aim to help you master a technique that will set you apart from most routine cosmetic treatment providers and enable you to step into the future of advanced cosmetic. Training THEORY will enable you to understand: Anatomy Vascular Supply, Nerves on the face Contraindications Patient consultation ONE-TO-ONE Training Nose Job Masterclass You will perform this procedure on live models under the supervision and guidance of highly experienced aesthetic practitioners. You will be trained under ENT specialist. We will give you all the knowledge you need for a safe technique in your practice. A certification of training will be provided upon completion of the course. You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. Additional information ATTENDANCE ONLINE (Theory), IN CLINIC (Practice) COURSE LEVEL EXPERT | Masterclass Course
WANs training course description A concise overview course covering Wide Area Networks with particular emphasis on the WAN options available including the use of the Internet. What will you learn Choose and evaluate WAN technologies. Recognise the role of service providers. Describe the benefits of VPNs. Describe how the Internet can be used as a WAN. Describe the equipment needed to connect LANS to WANS. List the speeds of various WAN technologies. WANs training course details Who will benefit: Anyone, although the course is particularly aimed at non-technical personnel needing some knowledge of WANS. Prerequisites: Network fundamentals Duration 1 day WANs training course contents WANS WAN architecture, Common WAN terms, Core vs access, service providers, relationship with 7 layer model, WAN equipment, how to choose a WAN. Layer 1 Copper, phone lines, fibre, coaxial, satellite, wireless. Cabling to the building, CPE cabling, interfaces. Layer 2 Dial up vs. Dedicated vs. packet switched networks and when to use them. Packet switching vs. circuit switching. Point to point and point to multipoint. Dialup access technologies Modems, ISDN, BRI, PRI. Access with dedicated lines XDSL, leased lines. WAN services X.25, SMDS, Frame Relay, CIR, ATM, Internet, MANS, dark fibre and other services. Case study: Selecting WAN technologies. Service provider technologies MPLS, SDH, WDM, DWDM. Routers Network addressing, default gateways, routing tables, routing protocols. Internet architecture Service providers, ISPs, private peering, public peering, core WANs in the Internet. VPNs Private networks, public networks, What are VPNs?, benefits of VPNs, tunnelling, encryption, IPSec. Case study: Specifying WAN connectivity.

M.D.D REJECTION THERAPY PACKAGE (SELF IMPROVEMENT)
4.9(27)By Miss Date Doctor Dating Coach London, Couples Therapy
Are you tired of feeling defeated and demotivated by rejection? Our Rejection Therapy Miss Date Doctor program is here to help you overcome your fear of rejection and take control of your life. Rejection can be a powerful force that holds us back from achieving our goals and realizing our full potential. Our program is designed to help you face your fears and develop the confidence and resilience you need to tackle any challenge that comes your way. With our Miss Date Doctor Rejection Therapy program, you’ll learn how to: Understand the root causes of your fear of rejection and develop strategies to overcome them Embrace rejection as a natural part of life and use it as a tool for personal growth Build your confidence and self-esteem through a series of exercises and challenges designed to push you out of your comfort zone Develop a growth mindset that will help you see every failure as an opportunity to learn and grow Become more resilient and adaptable in the face of adversity Our program is led by experienced coaches who have helped countless people overcome their fear of rejection and achieve their goals. With our expert guidance and support, you’ll be well on your way to developing the skills and mindset you need to succeed in all areas of your life. The Miss Date Doctor Rejection Therapy Package helps with the following areas: Rejection Therapy, fear of rejection, personal growth, confidence, self-esteem, growth mindset, failure, resilience, adaptability, experienced coaches. Improving the following areas in your life: defeated, demotivated, overcome, challenges, comfort zone, opportunity, adversity, skills, mindset, succeed. https://relationshipsmdd.com/product/rejection-therapy-package/

Certified Kanban System Design – KMP I - 30 Apr - 2 May
By Tom Reynolds
Attend our world class Kanban University accredited Certified Kanban System Design training course and learn to implement Kanban in your company

STGO Awareness, Abnormal Loads & Escort Vehicles Course - Online - November 2025
By Total Compliance
STGO Abnormal Loads

Secretary PA Training
By EduTech - Taylor Mason
During this 2 day course, you will develop a learning-based action plan to use in your workplace ensuring that you can put the learning into action.

Secretary PA Training
By EduTech - Taylor Mason
During this 2 day course, you will develop a learning-based action plan to use in your workplace ensuring that you can put the learning into action.
