- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
17547 Courses delivered On Demand
Diploma in Healthcare Assistant + Health and Social Care Support Training - CPD Accredited
4.8(9)By Skill Up
20-in-1 Diploma Bundle | Gifts: ! Free CPD PDF + Transcript | Lifetime Access

Security Threat Management (STM) Diploma: Advanced Cybersecurity & Threat Management
4.8(9)By Skill Up
Flash Sale! CPD Certified | 20-in-1 Premium Bundle | Free PDF & Transcript Certificate | Lifetime Access

IT Security: Cyber Security & CompTIA Network+ (AWS, Cisco ASA Firewall) - CPD Certified
4.8(9)By Skill Up
5 STAR Rated | CPD Certified Diploma | 22-in-1 Premium Bundle | 22 Free PDF+ Transcript Certificate | Lifetime Access

Introduction to the Personal Safety of 'Lone Workers' Approved Online Training
By Twig Services Ltd
Introduction to the Personal Safety of 'Lone Workers' Approved Online Training

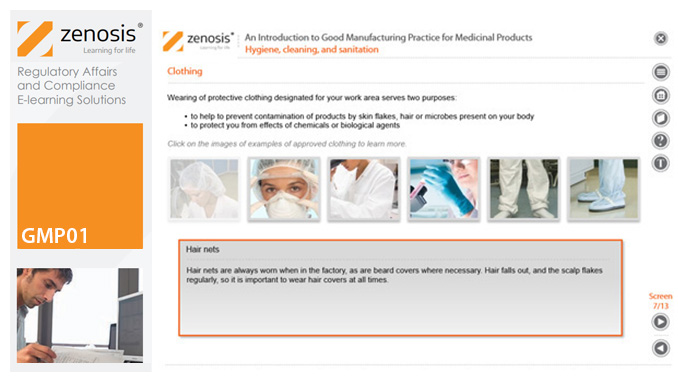
CT07: An Introduction to Clinical Trials and Drug Development
By Zenosis
This module provides an understanding of how clinical trials fit into the drug development process. It outlines the key historical events leading to the development of controlled clinical trials. It specifies the purpose of trials, outlines their features, and identifies codes and regulations that apply to them. Finally, it describes the environment of cost control in which the modern pharmaceutical industry operates.
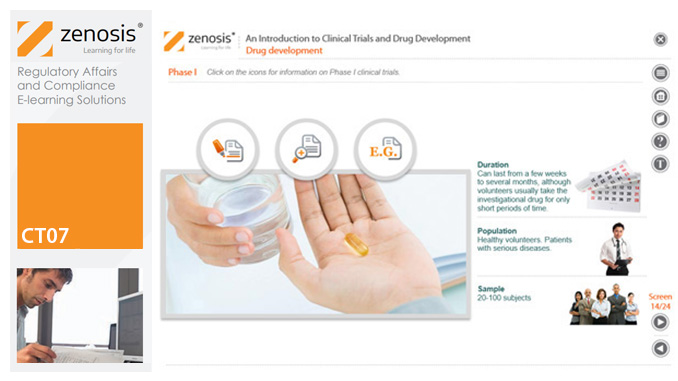
GMP01: An Introduction to Good Manufacturing Practice for Medicinal Products
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. The rules may be written into law or set out in guidance documents from regulatory authorities. Regulators will not allow medicinal products to be placed, or to remain, on the market in their country unless the products can be shown to be manufactured in compliance with GMP. To this end, they carry out inspections of manufacturing plants. Companies that persistently commit serious breaches of GMP requirements have suffered huge fines.