- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Social Responsibility (SR) Lead Auditor Training Course (ISO 26000:2010, SA8000:2014)
By TUVSW Academy
ISO 26000:2010 and SA8000:2014 are international standards of Social Responsibility/ Accountability management systems (SR), the existence of these requires competent personnel to interpret their requirements, address those to integrate SR in an organization, and audit the organization to assess the implementation and effectiveness of overall implementation. This course is designed for professionals who are responsible for any aspect of Social Responsibility as well as for those, specifically, who are pursuing their career in the field of auditing and certification. This course enhances the knowledge and skills of delegates to understand the standard and audit it effectively. The course is consisting of study material in the form of self-study typewritten and exercises. Attendees of this course will be, on completion, competent to implement SR, Plan, Conduct & Report 1st, 2nd & 3rd party audits. Features of Couse ✔ 100% Online Self-paced: Considering busy schedules, we have designed this course to be attended online without bounding with the timings. To make it interactive, we have created different communication groups where candidates can discuss the points with other fellows as well as the trainers of course. Also, they can interact with trainers on monthly demonstration classes. ✔ Testing the Learning: Each section of course is covered with exercise to check your understanding in real-time, and overall result is affected by exercises you complete. ✔ Self-Scheduling: This 40 Hours full fledge course is designed to match your schedule. You will get a life time access to this course and complete it on your ease. ✔ Superlative Material: The training is designed, developed and reviewed by competent auditors with extensive experience of auditing in different regions of world. ✔ Approved Course: The course is approved by one of the well-known personal certifying body “Exemplar Global”. Who should attend this? This course is recommended to be attended by those who are involved in the implementation and/ or auditing of Social Responsibility (SR), specifically Social Responsibility (SR) Managers, Auditors, and others willing to add credibility with a widely accepted qualification for auditing. Also, recommended to satisfy the applicable requirement of training and competence, if any. This course can add value to your profile if you are Intending to perform audits of Social Responsibility (SR). A Social Responsibility (SR) Executive/ Management Representative An Existing Internal Auditor A Social Responsibility (SR) Consultant Responsible for implementing the Social Responsibility (SR) standard. Responsibility to evaluate the outcome of internal Social Responsibility (SR) audits and have responsibility/ authority to improve the effectiveness of the Social Responsibility (SR). Pursuing to make a career in Social Responsibility (SR) auditing. Course Duration: 40 Learning Hours and extended time of exercise & Exam. Certificate: Those who pass all exercises with 50% at least in each exercise will be awarded with successful completion certificate with the approval of Exemplar Global and a Lifetime validity. Note: Each exercise have 2 retakes, if a candidate fails in all 3 terms, the course will be blocked there and the candidate will have to purchase it again by paying 20% of the original price. Content of Complementary Documentation Kit: 01 Social Responsibility Manual 08 Management System Procedures and 09 OHS Procedures 08 Policies 43 Forms 10 SOPs Language of Course: English

GLP01 - Good Laboratory Practice
By Zenosis
The purpose of GLP is to provide assurance of the quality and reliability of nonclinical study data. GLP covers the planning, performance, monitoring, recording and reporting of studies. Regulatory authorities typically require GLP rules to be followed for nonclinical studies intended to support an application for approval of clinical research or marketing of a product containing the test item. This course outlines the history of GLP and explains why it is important, identifies the penalties that may be incurred for noncompliance, and sets out requirements that need to be met. Learners are also referred to the two main sources of GLP rules: The Organisation for Economic Co-operation and Development’s Principles on Good Laboratory Practice and US Regulation 21 CFR 58: Good Laboratory Practice for Nonclinical Laboratory Studies.

The Ultimate Flask Course
By Packt
This course will show you how to build Python-based web applications using Flask. You will cover the basics of the Flask framework and learn how to add functionality to your Flask applications using the popular extensions.

Web Development Secrets Revealed - Critical Rendering Path, HTTP, AJAX, and More
By Packt
A comprehensive web development course that will help you understand 'why' things work and not just 'how'. Learn to write better code to boost your website traffic; know how to precision fix and tweak behavior and performance; improve your market penetration and your margins. Everything you need to know about the Critical Rendering Path, AJAX, and HTTP is right here at your fingertips.

Traibcert Awareness online course is a comprehensive package that delves into every nook and corner you deserve to know about ISO 22301, including all the erstwhile requirements and pragmatic impeccability for compliance. The course is durably defined for novices who exhibit great affinity towards Business continuity management and ISO standards.

Traibcert Awareness online course is a comprehensive package that delves into every nook and corner you deserve to know about ISO 27001, including all the erstwhile requirements and pragmatic impeccability for compliance. The course is durably defined for novices who exhibit great affinity towards Information security management and ISO 27001 standards.

SUB01: Orphan Drug Designation in the USA and Europe
By Zenosis
Medicines for the prevention, diagnosis, or treatment of rare diseases have become known as ‘orphan drugs’ because of their commercial unattractiveness. Development of such products is successfully encouraged through incentives offered by regulatory authorities. To qualify for important incentives, the sponsor of a drug must gain ‘orphan designation’ for its use in an indication. This module describes the requirements for orphan designation and how to apply for it in the USA and the European Economic Area.
SUB04: Preparing Submissions in the Common Technical Document (CTD) Format
By Zenosis
The CTD is the internationally recognised standard format for submissions to medicines regulatory authorities. In the European Economic Area, the USA and Canada, the CTD, in its electronic format (eCTD), is mandatory for all applications for marketing approval and all subsequent related submissions. The CTD is accepted in many other countries, being mandatory for new prescription medicines in some. This module explains the rationale for the CTD and provides guidance on its structure and format and the ways in which it is used.

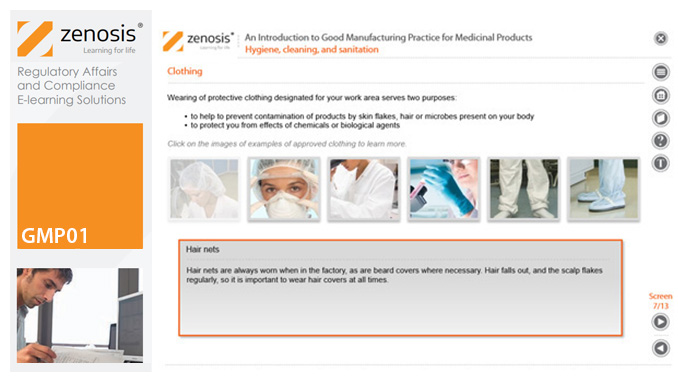
GMP01: An Introduction to Good Manufacturing Practice for Medicinal Products
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. The rules may be written into law or set out in guidance documents from regulatory authorities. Regulators will not allow medicinal products to be placed, or to remain, on the market in their country unless the products can be shown to be manufactured in compliance with GMP. To this end, they carry out inspections of manufacturing plants. Companies that persistently commit serious breaches of GMP requirements have suffered huge fines.