- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
180 Courses delivered On Demand
Explore the dynamic world of Fast-Moving Consumer Goods (FMCG) with our comprehensive course. Learn about marketing strategies, sales tactics, retailing, e-commerce, international markets, and industry trends. Dive into case studies, including Coca-Cola's marketing strategy, and gain insights to excel in the FMCG sector. Enroll now to stay ahead in this rapidly evolving industry.

Overview Gain compliance expertise, learn how to support deals and product development, and confidently inspect risk management, by taking the comprehensive Legal Consultant Diploma course. This advanced Legal Consultant Diploma provides you with a solid understanding of legal consulting, with an overview of a legal advisor's role, the UK's legal system and legal terminology. You'll learn how to transcribe, edit, and correspond with judges, the general principles of criminal law, property law, and employment law. Moreover, you'll gain professional legal writing, researching and proofreading skills, building your proficiency in Microsoft Office. Guarantee your professional development by enrolling today! How will I get my certificate? You may have to take a quiz or a written test online during or after the course. After successfully completing the course, you will be eligible for the certificate. Who is this course for? There is no experience or previous qualifications required for enrolment on this Legal Consultant Diploma. It is available to all students, of all academic backgrounds. Requirements Our Legal Consultant Diploma is fully compatible with PC's, Mac's, Laptop, Tablet and Smartphone devices. This course has been designed to be fully compatible on tablets and smartphones so you can access your course on wifi, 3G or 4G.There is no time limit for completing this course, it can be studied in your own time at your own pace. Career path Having these various qualifications will increase the value in your CV and open you up to multiple sectors such as Business & Management , Admin, Accountancy & Finance, Secretarial & PA, Teaching & Mentoring etc. Course Curriculum 4 sections • 42 lectures • 08:50:00 total length •Module 01: Legal Advice and Advisor: 00:25:00 •Module 02: Legal Adviser's Core Skills: 00:30:00 •Module 03: The Client: 00:25:00 •Module 04: Giving Legal Advice: Interviewing: 00:25:00 •Module 05: Giving Legal Advice: Recording and Preparing to Advise: 00:10:00 •Module 06: English Court System: 00:15:00 •Module 07: Property Law: 00:35:00 •Module 08: Employment Law: 00:35:00 •Module 09: Family Law: 00:35:00 •Module 10: Criminal Law: 00:20:00 •Navigate in Microsoft Word: 00:12:00 •Create and Save Word Documents: 00:24:00 •Manage Your Workspace: 00:06:00 •Edit Documents: 00:16:00 •Preview and Print Documents: 00:04:00 •Customize the Word Environment: 00:08:00 •Apply Character Formatting: 00:17:00 •Control Paragraph Layout: 00:19:00 •Align Text Using Tabs: 00:07:00 •Display Text in Bulleted or Numbered Lists: 00:03:00 •Apply Borders and Shading: 00:05:00 •Make Repetitive Edits: 00:06:00 •Apply Repetitive Formatting: 00:10:00 •Use Styles to Streamline Repetitive Formatting Tasks: 00:14:00 •Sort a List: 00:05:00 •Format a List: 00:06:00 •Insert a Table: 00:07:00 •Modify a Table: 00:06:00 •Format a Table: 00:03:00 •Convert Text to a Table: 00:04:00 •Insert Symbols and Special Characters: 00:04:00 •Add Images to a Document: 00:11:00 •Apply a Page Border and Color: 00:03:00 •Add Headers and Footers: 00:06:00 •Control Page Layout: 00:05:00 •Add a Watermark: 00:04:00 •Check Spelling Grammar and Readability: 00:07:00 •Use Research Tools: 00:06:00 •Check Accessibility: 00:03:00 •Save a Document to Other Formats: 00:04:00 •Mock Exam - Legal Consultant Diploma: 00:20:00 •Final Exam - Legal Consultant Diploma: 00:20:00

Delve into the intricacies of European medical device regulatory frameworks with our comprehensive course, 'Essentials of European Medical Device Regulations.' This meticulously structured program provides an in-depth exploration of the critical components and legislative requirements necessary for navigating the complex landscape of medical device regulations in Europe. The first module introduces the foundational aspects of the European regulations, setting the stage for a deeper understanding of the legal and procedural elements that govern this sector. As the course progresses, participants will gain a thorough grasp of the Essential Components of EU MDR (Module 2), the pivotal aspects of Reporting Requirements and Identification (Module 3), and the integral role of Quality Systems in medical device regulation (Module 4). This course is meticulously designed to cater to the needs of professionals seeking a robust understanding of the regulatory environment, ensuring they are well-equipped to adhere to and implement these critical regulations. Learning Outcomes Acquire a solid foundation in the basic principles and framework of European regulations on medical devices. Understand the key elements of the EU Medical Device Regulation (MDR) and their application in the industry. Gain insights into the specific requirements for reporting and identification within the European regulatory context. Learn about the implementation and management of quality systems in compliance with medical device regulations. Develop the ability to interpret and apply regulatory guidelines in professional settings, enhancing compliance and operational efficiency. Why choose this Essentials of European Medical Device Regulations course? Unlimited access to the course for a lifetime. Opportunity to earn a certificate accredited by the CPD Quality Standards and CIQ after completing this course. Structured lesson planning in line with industry standards. Immerse yourself in innovative and captivating course materials and activities. Assessments designed to evaluate advanced cognitive abilities and skill proficiency. Flexibility to complete the Course at your own pace, on your own schedule. Receive full tutor support throughout the week, from Monday to Friday, to enhance your learning experience. Unlock career resources for CV improvement, interview readiness, and job success. Who is this Essentials of European Medical Device Regulations course for? Professionals in the medical device industry seeking to deepen their understanding of EU regulations. Regulatory affairs specialists aiming to stay updated with the latest European legislative changes. Quality assurance personnel in the healthcare sector requiring knowledge of regulatory compliance. Medical device manufacturers and distributors needing to align their products with EU standards. Healthcare consultants and advisors focusing on European medical device regulatory frameworks. Career path Regulatory Affairs Manager: £40,000 - £60,000 Quality Assurance Specialist: £35,000 - £50,000 Compliance Officer: £30,000 - £45,000 Product Development Engineer: £33,000 - £55,000 Healthcare Consultant: £45,000 - £70,000 Medical Device Auditor: £37,000 - £53,000 Prerequisites This Essentials of European Medical Device Regulations does not require you to have any prior qualifications or experience. You can just enrol and start learning. This course was made by professionals and it is compatible with all PC's, Mac's, tablets and smartphones. You will be able to access the course from anywhere at any time as long as you have a good enough internet connection. Certification After studying the course materials, there will be a written assignment test which you can take at the end of the course. After successfully passing the test you will be able to claim the pdf certificate for £4.99 Original Hard Copy certificates need to be ordered at an additional cost of £8. Course Curriculum Module 1: Overview to European Regulations on Medical Devices Overview to European Regulations on Medical Devices 00:43:00 Module 2: Essential Components of EU MDR Essential Components of EU MDR 00:40:00 Module 3: Reporting Requirements and Identification Reporting Requirements and Identification 00:27:00 Module 4: Quality System in Medical Device Regulation Quality System in Medical Device Regulation 00:36:00

Sale Ends Today Production Operative Training Admission Gifts FREE PDF & Hard Copy Certificate| PDF Transcripts| FREE Student ID| Assessment| Lifetime Access| Enrolment Letter Did you know that the logistics and warehousing industry in the UK employs over 1.7 million people? The sector is booming, driven by the surge in e-commerce and global trade. The Production Operative Training bundle is your gateway to mastering the complex logistics that power businesses across the nation. Get ready to step into a career that's at the heart of every major industry, ensuring that products move efficiently from manufacturing to the hands of the consumer! The Production Operative Training course offers a comprehensive curriculum designed to equip you with the knowledge and skills required for a successful career in production and operations management. From the essentials of Warehouse Management to the intricacies of International Trade and Logistics Management, this bundle covers a wide range of topics crucial to the field. You will learn about Inventory Management, Warehouse Safety, Transport Management, and Quality Management, including ISO 9001. Each course is tailored to enhance your understanding of the physical and administrative aspects of warehouse and operations management, ensuring you're well-prepared for the challenges of the industry. Courses Are Included in this Bundle: Course 01: Production Operative Training Course 02: Product Management Course 03: Fundamentals of Warehousing and Storage Course 04: Warehouse Management Course 05: Diploma of Import/ Export (International Trade) & Logistics Management Course 06: Operations and Warehouse Management with Transport Management Course 07: Manual Handling for Warehouse Operation Course 08: Inventory Management Course 09: Warehouse Safety Course 10: Warehouse Operative Course 11: Warehouse Associate Course 12: Material Management Course 13: Logistics Management Course 14: Procurement and Supply Chain Management Course 15: Transport And Logistics Course 16: Transport Manager: Successful Transport Management Course 17: Transport Planning Course 18: Import/Export Processing Course 19: Delivery Manager Course 20: Quality Management and Strategic Training - ISO 9001 Course 21: Effective Organisational Reporting Course 22: Forklift Training Course 23: RIDDOR Training Course 24: Ladder Safety Course 25: LOLER Training Course 26: Emergency First Aid at Work Course 27: Project Management: Six Sigma Course 28: Time Management Skills Course 29: Concussion and Brain Injury Awareness Course 30: Effective Communication Training Level-3 Take charge of your future by enroling in our Production Operative Training. This program is designed to catapult you into a pivotal role within the logistics and warehousing sector. These courses arm you with the essential tools and insights needed to excel. Enrol now and start crafting a successful future in an industry that supports global commerce! Learning Outcome of this Bundle: Understand key principles of warehouse and inventory management. Gain expertise in international trade and logistics operations. Master the use of forklifts and safety procedures in warehousing. Develop skills in strategic quality management and ISO 9001. Learn effective project and time management techniques. Enhance communication and report-writing skills for organisational success. With a single payment, you will gain access to the Production Operative course, including 10 premium courses, a QLS Endorsed Hardcopy certificate (for the title course) and 11 PDF certificates for Absolutely free. Why Choose Us? Get a Free QLS Endorsed Certificate upon completion of Logistics Management Get a Free Student ID Card with this training program (£10 postal charge will be applicable for international delivery) The course is Affordable and Simple to understand Get Lifetime Access to the course materials The training program comes with 24/7 Tutor Support Start your learning journey straight away! The Production Operative Training course is expertly designed to provide learners with a robust foundation in warehouse and inventory management. This course offers a deep dive into the operational techniques and strategies that are critical for maintaining efficiency and accuracy in the storage and handling of goods. Participants will learn to optimise space, manage stock levels effectively, and implement cutting-edge inventory systems that minimise errors and enhance operational performance. Additionally, learners will acquire specialised skills in international trade and logistics operations. This includes mastering complex logistical frameworks that govern global trade, understanding customs regulations, and managing international supply chains. The course also emphasises the importance of safety and proficiency in equipment handling, where learners will master the use of forklifts and other essential warehouse machinery, ensuring compliance with health and safety standards. Moreover, this diploma offers learners the opportunity to acquire skills that are highly valued in the field of Production Operative. With this Certification, graduates are better positioned to pursue career advancement and higher responsibilities within the Production Operative setting. The skills and knowledge gained from this course will enable learners to make meaningful contributions to Production Operative related fields impacting their Production Operative experiences and long-term development. Course Curriculum Course 01: Production Operative Training Module 01: Understanding Facilitation Module 02: Process Vs. Content Module 03: Laying The Groundwork Module 04: Tuckman And Jensen's Model Of Team Development Module 05: Building Consensus Module 06: Reaching A Decision Point Module 07: Dealing With Difficult People Module 08: Addressing Group Dysfunction Module 09: About Intervention Module 10: Intervention Techniques Course 02: Product Management Module 01: Introduction To Product Management Module 02: Product Classification Module 03: Developing The Product Plan Module 04: New Product Development Module 05: Levels Of A Product And Product Life Cycle Module 06: Product Pricing Strategy Module 07: Product And Brand Portfolio Analysis Module 08: Channels Management Module 09: Basics Of Marketing For Products Module 10: Financial Analysis For Product Management Course 03: Fundamentals of Warehousing and Storage Module 01: Stockroom and Warehousing Module 02: Processes in Warehousing and Stockroom Management Module 03: Order Picking System in Warehouses Module 04: Logistics and Supply Chain in a Warehouse Module 05: Classification of Inventory in the Warehouse Module 06: Inventory Management Techniques Module 07: Technology in the Warehouse Module 08: Adopting the Right Technology Module 09: Core Functions of a Warehouse Management System (WMS) Module 10: Cost Management Module 11: Health & Safety Management Module 12: Workforce Management Module 13: Fire and Electrical Safety Management Module 14: Storage Systems Module 15: Automated Storage and Retrieval Systems =========>>>>> And 27 More Courses <<<<<========= How will I get my Certificate? After successfully completing the course, you will be able to order your QLS Endorsed Certificates and CPD Accredited Certificates as proof of your achievement. PDF Certificate: Free (Previously it was £12.99*30 = £390) CPD Hard Copy Certificate: Free (For The First Course: Previously it was £29.99) QLS Endorsed Hard Copy Certificate: Free (For The Title Course: Previously it was £119) CPD 300 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone interested in learning more about the topic is advised to take this bundle. This bundle is ideal for: Aspiring logistics professionals. Warehouse managers. Supply chain graduates. Operations staff. Future transport managers. Production operatives. Requirements You will not need any prior background or expertise to enrol in this bundle. Career path After completing this bundle, you are to start your career or begin the next phase of your career. Warehouse Manager: $30,000 - $50,000 Logistics Coordinator: $25,000 - $45,000 Supply Chain Analyst: $35,000 - $60,000 Transport Manager: $40,000 - $65,000 Quality Assurance Manager: $40,000 - $70,000 Forklift Operator: $20,000 - $35,000 Certificates CPD Accredited Digital Certificate Digital certificate - Included CPD Accredited e-Certificate - Free Enrolment Letter - Free Student ID Card - Free CPD Accredited Hard Copy Certificate Hard copy certificate - Included Please note that International students have to pay an additional £10 as a shipment fee. Diploma in Logistics Management at QLS Level 5 Hard copy certificate - Included Please note that International students have to pay an additional £10 as a shipment fee.

Unveil the secrets of the beauty world with 'Creating Organic Cosmetics: Formulation and Production'. In an era where natural makeup and organic cosmetics are revolutionising the industry, this course offers an in-depth exploration into the art of crafting these products. Delve into the science of skin, understand the role of natural ingredients, and master the formulation of facial, body, and hair care products. As you progress, you'll gain insights into the preservation, stability, and quality control of organic makeup, ensuring safety and efficacy in every creation. Learning Outcomes Comprehend the foundational principles of organic cosmetics formulation. Recognise the significance of skin science in product development. Identify and utilise natural ingredients effectively in organic cosmetics. Formulate a diverse range of organic skincare, body, and hair care products. Understand the intricacies of quality control, safety assessment, and marketing of organic cosmetics. Why buy this Creating Organic Cosmetics: Formulation and Production? Unlimited access to the course for a lifetime. Opportunity to earn a certificate accredited by the CPD Quality Standards and CIQ after completing this course. Structured lesson planning in line with industry standards. Immerse yourself in innovative and captivating course materials and activities. Assessments designed to evaluate advanced cognitive abilities and skill proficiency. Flexibility to complete the Course at your own pace, on your own schedule. Receive full tutor support throughout the week, from Monday to Friday, to enhance your learning experience. Unlock career resources for CV improvement, interview readiness, and job success. Who is this Creating Organic Cosmetics: Formulation and Production for? Individuals passionate about organic and natural beauty products. Entrepreneurs aiming to launch their organic cosmetics brand. Cosmetic formulators keen on expanding into the organic niche. Marketing professionals in the beauty sector. Anyone interested in the science and art of organic makeup formulation. Career path Organic Cosmetics Formulator: £28,000 - £45,000 Natural Makeup Brand Founder: £30,000 - £60,000 (varies with business success) Quality Control Specialist in Cosmetics: £25,000 - £40,000 Organic Skincare Specialist: £27,000 - £42,000 Cosmetic Marketing Manager: £35,000 - £55,000 Organic Hair Care Product Developer: £26,000 - £43,000 Prerequisites This Creating Organic Cosmetics: Formulation and Production does not require you to have any prior qualifications or experience. You can just enrol and start learning. This course was made by professionals and it is compatible with all PC's, Mac's, tablets and smartphones. You will be able to access the course from anywhere at any time as long as you have a good enough internet connection. Certification After studying the course materials, there will be a written assignment test which you can take at the end of the course. After successfully passing the test you will be able to claim the pdf certificate for £4.99 Original Hard Copy certificates need to be ordered at an additional cost of £8. Course Curriculum Module 1: Introduction to Organic Cosmetics Formulation Introduction to Organic Cosmetics Formulation 00:21:00 Module 2: Skin Science Skin Science 00:25:00 Module 3: Natural Ingredients in Organic Cosmetics Natural Ingredients in Organic Cosmetics 00:33:00 Module 4: Organic Facial Skincare Products Organic Facial Skincare Products 00:36:00 Module 5: Organic Body Care Products Organic Body Care Products 00:21:00 Module 6: Organic Hair Care Products Organic Hair Care Products 00:19:00 Module 7: Organic Makeup Products Organic Makeup Products 00:33:00 Module 8: Preservation and Stability of Organic Cosmetics Preservation and Stability of Organic Cosmetics 00:23:00 Module 9: Quality Control and Safety Assessment Quality Control and Safety Assessment 00:21:00 Module 10: Advanced Organic Cosmetics Formulation Advanced Organic Cosmetics Formulation 00:21:00 Module 11: Marketing and Branding Organic Cosmetics Marketing and Branding Organic Cosmetics 00:30:00

Unleash the captivating world of fragrance creation with our exclusive Perfume Mastery Course. Dive into the art of perfumery, where each essence tells a story, and every note ignites emotions. Begin your aromatic journey with an insightful introduction to the enchanting realm of perfumery, laying the foundation for your olfactory odyssey. Discover the secret alchemy of perfume ingredients, unraveling the aromatic symphony that captivates the senses. Immerse yourself in the delicate dance of crafting scents, as you learn the intricacies of the perfume-making process, unlocking the magic behind each captivating bottle. Key Features: CPD Certified Free Certificate Developed by Specialist Lifetime Access Delve into the fine art of perfume testing and evaluation, honing your ability to discern the nuances that make a fragrance unforgettable. Navigate the essential terrain of perfumery safety and regulations, ensuring your creations meet industry standards. Master the control of substances hazardous to health, safeguarding both your art and those who experience it. Finally, explore the exciting prospect of starting your own perfume business, turning passion into a fragrant venture. Elevate your aromatic expertise, and let the alluring world of perfumery unfold before you. Course Curriculum Module 01: Introduction to Fragrance Artistry Module 02: Fragrance Components Module 03: Fragrance Crafting Process Part 1 Module 04: Fragrance Crafting Process Part 2 Module 05: Fragrance Assessment and Review Module 06: Fragrance Safety and Compliance Module 07: Management of Hazardous Substances (MOHS) Module 08: Initiating Your Own Fragrance Enterprise Learning Outcomes: Analyze diverse perfume ingredients for nuanced fragrance compositions. Execute the intricate steps of the perfume-making process with precision. Evaluate and refine perfumes to achieve exceptional olfactory experiences. Prioritize safety measures in compliance with perfumery regulations. Demonstrate proficiency in controlling hazardous substances for fragrance production. Develop a strategic roadmap for initiating and managing a successful perfume business. CPD 10 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Aspiring perfumers seeking in-depth knowledge of fragrance creation. Entrepreneurs with a passion for entering the perfume industry. Cosmetic professionals aiming to expand their product development expertise. Individuals fascinated by the art and science of perfumery. Artisans and craft enthusiasts eager to master the alchemy of fragrance creation. Career path Fragrance Chemist Perfume Product Developer Regulatory Compliance Specialist (Perfumery) Fragrance Evaluator Independent Perfumer Boutique Perfume Business Owner Certificates Digital certificate Digital certificate - Included Certificate of Completion Digital certificate - Included Will be downloadable when all lectures have been completed.

Mastering Market Insights: A Comprehensive Course on Market Research
By Xpert Learning
About Course Unlock key insights with our comprehensive guide to market research - propel your business towards unprecedented growth Our immersive online course on Market Research provides a comprehensive exploration into the intricate art and science of understanding consumer behaviour, trends, and market dynamics. Do you want to grow your business and take it to new heights? Achieving sustainable growth requires a deep understanding of your target audience and a keen awareness of industry trends. This marketing course equips you with the knowledge and skills required to understand your target customers and their needs. We start with a brief history and definition of 'market research' before we lay out the six steps of the market research process. This course examines the classifications of market research and explains how to identify research problems to set up the market research process. We show you how to develop and design a market research approach. We also trace the relationship between market problems and research design by distinguishing between exploratory, descriptive and causal research. The course goes over data analysis techniques and discusses how to deliver effective presentations of research findings to facilitate analysis and discussion. We cover qualitative and quantitative market research methods and provide examples to help you with questionnaire design with examples of open- and closed-end questions. We demonstrate data analysis techniques to help you leverage your research findings to make better business decisions. Finally, the course explains how to communicate research results while adhering to market research ethics. This course has no prerequisites and suits anyone interested in business and marketing. It is particularly useful for small business owners, entrepreneurs and marketers and helps you to create focused marketing campaigns that connect with your audience to boost customer engagement, brand loyalty and business success. Sign up to learn how to conduct market research that lets you understand consumer behaviour, spot gaps in the market and grow your business. Whether you're a budding marketer, a business owner, or a student of the commerce field, this course offers a comprehensive and practical guide to mastering market research. Enroll now to gain the knowledge and skills that can help shape business strategies, influence product development, and drive business success. What Will You Learn? Discuss the importance of market research in supporting marketing decision-making Identify the factors that influence the decision to conduct market research Outline the key components of a market research proposal Discuss the significance of problem identification in marketing research Analyse the key characteristics and advantages of qualitative research Recognise the factors that affect the sample size and accuracy in quantitative research Identify the key components and goals of a questionnaire Explain the importance of data validation and quality control Summarise the significance of the report and presentation as key components of marketing research Discuss the ethical principles of research methodology and the importance of integrity in market research Course Content Introduction to Market Research Introduction to Market Research The Market Research Process The Market Research Process Market Research Design Market Research Design Defining the Problem & Developing Research Approach Defining the Problem & Developing Research Approach Qualitative Market Research Qualitative Market Research Quantitative Market Research Quantitative Market Research Questionnaire Design Questionnaire Design Data Analysis Data Analysis Communicating Research Results Communicating Research Results Research Ethics Research Ethics Conclusion Conclusion A course by Xpert Learning Audience Marketers Current and aspiring product managers Business executives Consultants Entrepreneurs

Description: If you have always felt like you can work for yourself and develop an organization by yourself, you have found the right place with our intrapreneurship and entrepreneurship course. With job security vanishing day by day, most skilled workers are looking for ways to channel their effort towards creating a startup by themselves. In this course, you will learn about the significance of entrepreneurship. You will learn about the features of an entrepreneur as well as study to evaluate your individual strengths. You will get to assess your individual entrepreneurial eligibility and display an outline as well as a measure of an innovative business idea. Moreover, this course will help you to understand various kinds of business ownership and structures. Learning Outcomes: Realise the significance of entrepreneurship for the most recent economy Figure out the features of an entrepreneur as well as learn evaluating your individual strengths Produce an entrepreneurial team in your organization Realise the system of entrepreneurship Devise a new product or process concept Figure out the worth of a sales plan Generate a start-up financial statement Evaluate your individual entrepreneurial eligibility Display an outline as well as assess a business product idea Recognise the target market and customers Improvise your value proposition Realise various kinds of business ownership and structures Assess franchising as well as business purchasing opportunities Assess franchising and business purchasing opportunities Generate the major business planning documents Produce financial projections for the business as well as gather funding Promote a product development plan, marketing plan, as well as sales plans Locate the routes to safeguard your intellectual asset Narrate proficient paths to brand the product Commence and develop your business Examine the behaviors of an entrepreneurial leader Figure out the proper resources to assist you for your journey Assessment: At the end of the course, you will be required to sit for an online MCQ test. Your test will be assessed automatically and immediately. You will instantly know whether you have been successful or not. Before sitting for your final exam you will have the opportunity to test your proficiency with a mock exam. Certification: After completing and passing the course successfully, you will be able to obtain an Accredited Certificate of Achievement. Certificates can be obtained either in hard copy at a cost of £39 or in PDF format at a cost of £24. Who is this Course for? Intrapreneurship and Entrepreneurship is certified by CPD Qualifications Standards and CiQ. This makes it perfect for anyone trying to learn potential professional skills. As there is no experience and qualification required for this course, it is available for all students from any academic background. Requirements Our Intrapreneurship and Entrepreneurship is fully compatible with any kind of device. Whether you are using Windows computer, Mac, smartphones or tablets, you will get the same experience while learning. Besides that, you will be able to access the course with any kind of internet connection from anywhere at any time without any kind of limitation. Career Path After completing this course you will be able to build up accurate knowledge and skills with proper confidence to enrich yourself and brighten up your career in the relevant job market. Module 1 Why Is Intrapreneurship Important? 00:15:00 Characteristics of Intrapreneurs 00:15:00 Picking Your Team 00:15:00 Becoming an Intrapreneur 00:15:00 Creating and Selling Your Ideas 00:30:00 The Implementation Plan 00:15:00 You an Intrapreneur? 00:15:00 Module 2 What It Takes to Make It 00:15:00 Resources to Consider 00:30:00 Laying the Groundwork 00:45:00 Building On Your Business Idea 00:45:00 Business Ownership Options 00:30:00 Key Documents to Prepare 01:00:00 Gathering Funding 00:00:00 Developing Your Product 00:30:00 Creating a Sales and Marketing Strategy 00:00:00 Branding 101 00:30:00 Setting Up Your Office 00:15:00 Launching the Business 00:30:00 Keeping the Business Moving 00:31:00 Being an Entrepreneurial Leader 00:15:00 Bringing It All Together 00:18:00 Certificate and Transcript Order Your Certificates and Transcripts 00:00:00

ICT02: Assuring Data Integrity in the Manufacture of Medicinal Products
By Zenosis
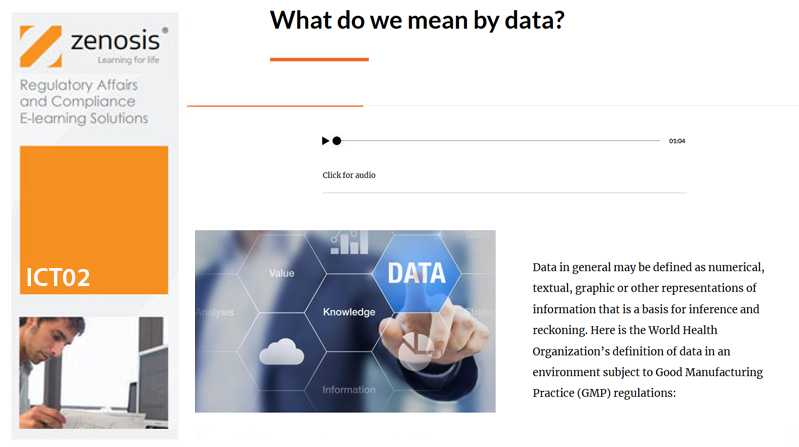
Pharmaceutical and biotechnology companies and researchers need to assure regulatory authorities of the reliability of the data that they generate or acquire during product development and manufacturing – that is, to demonstrate data integrity. Data integrity is assessed during regulatory inspections of manufacturing and research sites. Inadequacies of data integrity are frequently reported by inspectors and result in regulatory actions against the companies or individuals concerned. Practices that assure data integrity are required by law and/or expected by regulators in the fields of nonclinical and clinical research, manufacturing and distribution, and pharmacovigilance of medicinal products. This course explains the requirements and describes principles and practices that should be followed to assure regulators and contractual partners of data integrity in the manufacture of medicinal products.