- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
1885 Courses delivered Live Online
Introduction to Good Manufacturing Practice
By Research Quality Association
Course Information This course offers foundational guidance and practical support tailored for individuals operating within Good Manufacturing Practice (GMP) frameworks. Explore the fundamental prerequisites of a pharmaceutical quality system (PQS) and delve into the application of quality risk management (QRM) principles, aligning with current regulations and guidance. Gain insights into pivotal aspects such as requirements, roles, and responsibilities, encompassing change control, document management, and key documentation essential for effective implementation of GMP with a focus on regulatory inspections and common findings. Is this course for you? Ideal for professionals engaged in GMP across various sectors, including: Research and Development (R&D) Contract Manufacturing Organisations Manufacturing Units Quality Control (QC) Laboratories Auditing Roles. What will you learn? Event objectives - by the end of the course, delegates shall: Have an awareness of the basic requirements of GMP Be aware of UK and EU GMP Rules and Guidance and relevant publications Understand the roles and responsibilities associated with GMP Be able to contribute to and maintain quality documentation Have a basic understanding of product lifecycle and manufacturing Understand the requirements of GMP in the QC laboratory context Have a basic understanding of risk management and mitigation principles Understand the need for quality systems and quality assurance activities Be aware of common regulatory findings. Learning outcomes: delegates will be able to: Implement their role within GMP with confidence and knowledge of the principle requirements Contribute effectively to the GMP quality system and their organisation’s compliance Comprehend where their organisation’s activities sit within the larger GMP arena Know where to seek further information within the published rules and guidance, UK Legislation, European Commission Directives, ICH Guidance and other relevant publications, as well as via the internet. Tutors Tutors will be comprised of (click the photos for biographies): Louise Handy Director, Handy Consulting Ltd Programme Please note timings may be subject to alteration. Day 1 09:30 Introductions and Scope of the Course Understand the group requirements and the tutor's background and experience. 09:45 Background and Regulatory Environment Setting the scene, understanding the context, key legislation. 10:30 Principles of GMP Key points and requirements. 11:15 Break 11:30 Personnel and Responsibilities Management and staff, duties and accountabilities. 12:00 Overview of GMP Manufacturing Basics of the product life cycle. 12:30 Lunch 13:15 Risk Management Workshop Practical exploration of risk and mitigation activities. 14:30 QC Laboratories Activities and practicalities. 15:15 Break 15:30 Compliance Quality Assurance and Self Inspection. 16:15 Question Time A chance for questions on the practicalities of GMP. 16:30 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Learn

Audit Programmes and Risk Assessment
By Research Quality Association
Course Information This one day course is designed to provide you with comprehensive guidance and practical help for when designing and implementing audit programmes. Using the guidance of ISO 19011 with reference to PV, GCP, GMP and GLP audit programmes, you will explore audit programme design, operation, review and improvement. The course will work through why audits are important and understanding the drivers behind a good audit programme. It will discuss how to identify and assess the risks in your organisation, linking them with organisational goals, using these risks as a basis for the design of a risk-based audit programme during facilitated practical workshops. Delegates will have the opportunity to consider and discuss common issues and constraints that may shape their audit programmes. By the end of the course you will have: A clear understanding of the role of audit programmes in managing compliance and of the drivers and risks behind audit programmes An understanding of the roles and responsibilities of management and personnel An appreciation of resourcing implications and auditor attributes A good insight into the practicalities and activities required for design of risk based audit programmes A comparison of your circumstances, challenges, common issues and ways to approach managing audit programmes with other delegates on the course. Tutors Tutors will be comprised of (click the photos for biographies): Louise Handy Director, Handy Consulting Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:15 Why do we Need to Audit? Exploring risk and regulation, responsibility and performance. 09:45 Discussion - Programmes, Needs, Commonalities Understanding the needs of delegates' own organisation and comparing common themes and threats. 10:00 Establishing an Audit Programme Design, objectives, risk and resources. This session discusses the consideration when designing audit programmes. 10:30 Break 10:45 Risk Management Considerations, guidance and methods for assessing and controlling risk. 11:00 Workshop 1 - Risks, Prioritisation and Control Looking at specific risks, assessing and evaluating to feed into audit programme management. 12:00 Workshop 1 - Feedback 12:30 Lunch 13:30 Putting it into Practice Resources, practicalities and challenges - the realities of auditing, including selection of auditors, ensuring practice will meet expectations and the reasons to note audit results. 13:45 Workshop 2- Designing Audit Programmes Designing audit programmes using output from risk assessment process. Challenges and flexibility. 15:00 Break 15:15 Workshop 2 - Feedback 15:30 Monitoring, Reviewing and Improving Why or when should existing processes change, understanding the implications of change or inaction and exploring how to improve the audit programme. 16:15 Panel Session This final session will address any outstanding issues raised by delegates. 16:30 Close of Course Extra Information Remote Course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device< Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Develop

Good Laboratory Practice Refresher and Hot Topics
By Research Quality Association
Course Information Join us for a comprehensive refresher focusing on crucial Good Laboratory Practice (GLP) requirements, including an emphasis on data integrity, recent developments, and emerging trends gleaned from MHRA inspections. The programme dives into specific domains such as risk assessment, OECD guidance on sponsor influence, and the advisory from OECD on QA. Additionally, delegates can benefit from a dedicated GLP clinic, facilitating discussions on understanding and upholding GLP compliance. Is this course for you? This course is tailored for study directors, principal investigators, test facility management, and QA professionals seeking to refresh their knowledge and responsibilities within the GLP framework. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Registration, Welcome and Introduction 09:20 Development of Good Laboratory Practice A reminder of the history of GLP, its current scope and application, with a synopsis of current UK, European and international standards. 09:50 Roles and Responsibilities of Study Director, Test Facility Management, Principal Investigator, Test Site Management, Study Staff and QA A reminder of the roles and responsibilities with regard to the GLP management and oversight of the Test Facility and the management and control of the study, as defined by GLP. 10:30 Break 10:45 Workshop 1 Workshop 1 Roles and responsibilities 11:15 Influence of Sponsors The published OECD Position Paper No. 21 regarding Possible Influence of Sponsors on conclusions of GLP Studies is reviewed and discussed. 11:45 Data Integrity The fundamentals of data integrity according to the OECD Guidance No. 22 on Data Integrity is discussed along with the responsibilities of Study Director, Test Facility Management, and study staff in ensuring the integrity of the GLP study data. 12:30 Lunch 13:15 Quality Assurance and GLP OECD Advisory No. 23 (Revision of OECD No.4)- A walk through of the changes to the OECD Guidance on the role and activities of Quality Assurance 13:45 Quality Improvement Tools and GLP The tools that might be considered for GLP and their role and operation when used in Test Facilities- OECD Position Paper No.24 published July 2022 14:15 Workshop 2 Workshop 2 Change control 14:30 Risk Assessment How should we assess risk and how can we use the process to assist in evaluation audit findings? 15:00 Break 15:15 Current hot topics in GLP Explore the current issues in Industry and trends /types of Regulatory inspection findings 15:50 GLP Clinic An opportunity to discuss any other issues regarding understanding and maintaining GLP Compliance. 16:30 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Learn

LOOKING FOR: ADULT FICTION, NON-FICTION Una is a Rights Agent at Susanna Lea Associates and am starting to build a client list. She grew up in London and graduated from the University of Cambridge with a BA in English in 2021. At university, Una was particularly interested in contemporary West African and South Asian literature. She started as an agent's assistant at SLA London in 2022 and now handles some translation rights and provides support with editorial work and submissions. Una's favourite reads always set her at ease right away and draws her in with impressive and accessible storytelling, memorable characters or an original hook. Una enjoys writing which expands her worldview, makes her laugh out loud or keeps her on her toes with its twists and turns. She welcomes submissions from debut authors and would be keen to read across a wide range of genres and styles. She is looking for literary, upmarket and book club fiction, and is always drawn to stories that explores the challenges and complexities of love and relationships in all its forms —within families, between friends or in romantic relationships. Una loves the way in which familial relationships are dissected in The Wren, The Wren by Anne Enright and the impact of the local community in Small Worlds by Caleb Azumah Nelson. She is always drawn to novels with a strong sense of community, whether they are unified by geography, culture, or in other unexpected ways. Una has always loved reading international and translated fiction and welcomes submissions from authors writing contemporary fiction that engages with cultures and traditions from around the world, such as in voices of the deities in Freshwater by Akwaeke Emezi or the subversive tales of Sayaka Murata. She is not looking for children’s or YA titles, science fiction or high-concept fantasy, but is open to submissions from authors writing in the speculative fiction space, and books with fantastical or otherworldly elements, be that magical realism or supernatural horror, such as Mona Awad's campus novel with a twist, Bunny. Una would also love to read more writing that engages with the natural world and reminds us of the limitations of human understanding. In non-fiction, Una enjoys books that furthers her understanding of wider societal issues — nature and the environment, culture, and little-known history. Her recent non-fiction favourites are Eve by Cat Bohannon, Doppelgänger by Naomi Klein and Unearthed by Claire Ratinon. Una would like you to submit a covering letter, 1 page synopsis and the first three chapters or 5,000 words of your manuscript in a single word document. (In addition to the paid sessions, Una is kindly offering one free session for low income/under-represented writers. Please email agent121@iaminprint.co.uk to apply, outlining your case for this option which is offered at the discretion of I Am In Print). By booking you understand you need to conduct an internet connection test with I Am In Print prior to the event. You also agree to email your material in one document to reach I Am In Print by the stated submission deadline and note that I Am In Print take no responsibility for the advice received during your agent meeting. The submission deadline is: Monday 22nd September 2025

Kick Start Your Career with CompTIA's Data Analysis Certification - Live Classes
5.0(1)By Media Tek Training Solutions Ltd
Get job ready with CompTIA's Data Analysis Certification. Live Classes - Career Guidance - Exam Included.

DTP Online Mentorship
By Draw to Perform
https://linktr.ee/drawtoperform

ONE SPACE LEFT! 29th September Rebeka Finch #Agent121. Looking for: NA, ADULT FICTION
5.0(3)By I Am In Print
LOOKING FOR: ADULT FICTION, NEW ADULT Rebeka Finch is a Junior Agent at the Darley Anderson Literary Agency. Alongside assisting Darley on his list of authors, Rebeka is building her own list of romance and romantasy authors, specifically for the BookTok hungry new adult/20+ markets. She is on the hunt for messy, cheesy, heartbreaking, relatable, characters and stories with romance at the very heart of the narrative. For those writing romantasy, Rebeka is looking for books that strike the perfect balance of romance, pace, action and world-building, with series potential. Importantly, she looks for strong, diverse, brave and relatable characters in these settings. People that feel like both a friend, a champion and an ally. Rebeka would like you to submit a covering letter, 1 -2 page synopsis and the first 5,000 words of your completed manuscript in a single word document. (In addition to the paid sessions, Rebeka is kindly offering one free session for low income/under-represented writers. Please email agent121@iaminprint.co.uk to apply, outlining your case for this option which is offered at the discretion of I Am In Print). By booking you understand you need to conduct an internet connection test with I Am In Print prior to the event. You also agree to email your material in one document to reach I Am In Print by the stated submission deadline and note that I Am In Print take no responsibility for the advice received during your agent meeting. The submission deadline is: Monday 22nd September 2025

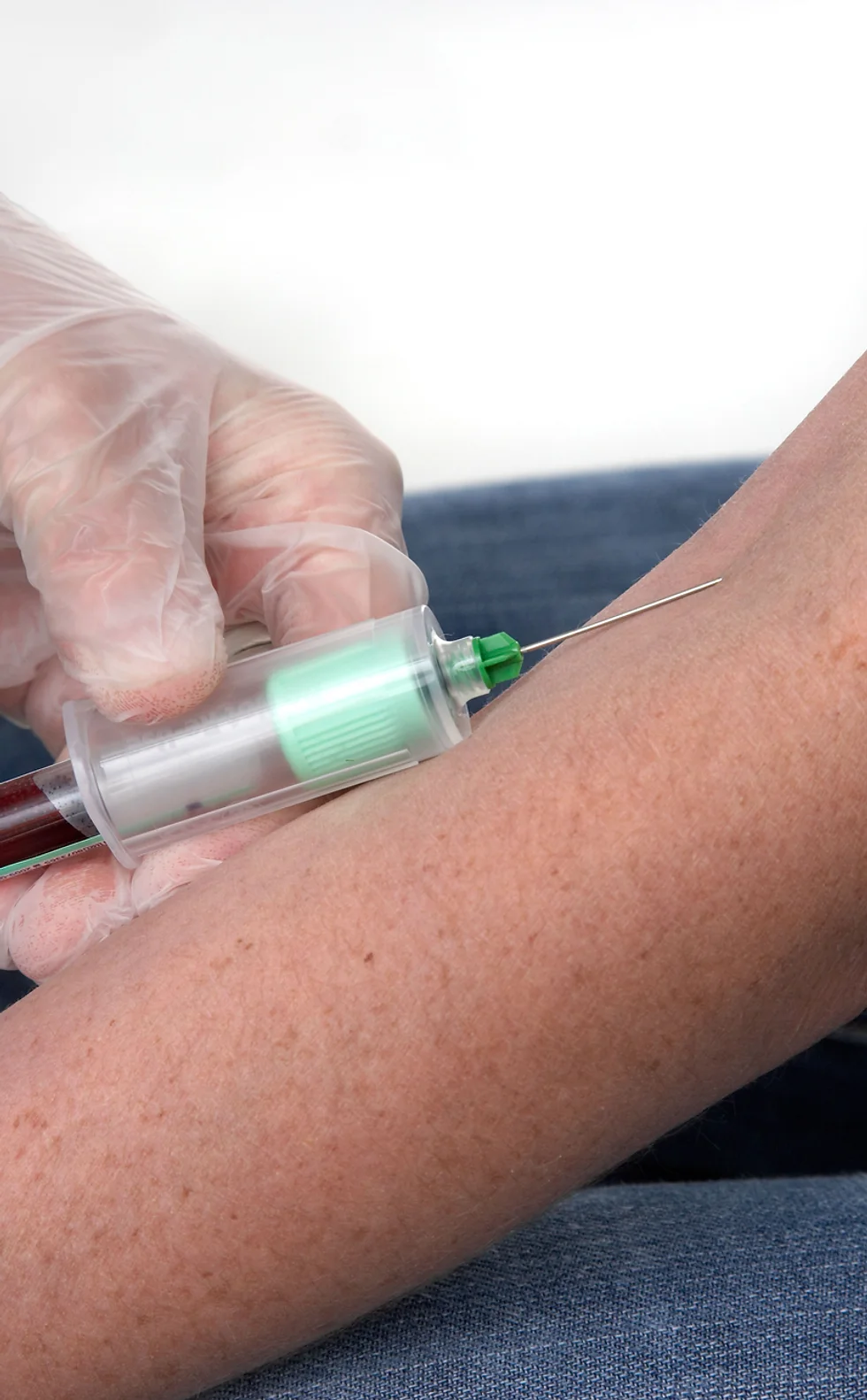
Introduction to Phlebotomy Course - Certificate Update and Renewal (GPT003R)
4.6(39)By Geopace Training
Update and renewal option for Geopace Training Certificate holders Update and renew your existing certificate ... Recommended certificate renewal after 18 months Nationally Recognised Dual Accreditations Open College Network Accredited (Advanced Level) CPD Accredited (The CPD Certification Service) Recaps phlebotomy theory incl. legislation Updates your certification and compliancy Awards cumulative credits and CPD points awarded Basic understanding of English language required Download a certificate on completion of your online course APPLICANTS ARE REQUIRED TO HAVE COMPLETED AN ACCREDITED INTRODUCTION TO PHLEBOTOMY COURSE