- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
5407 Courses in Cardiff delivered Live Online
ISO 45001 Internal Auditor
By Training Centre
ISO 45001 is the first global occupational health and safety management system standard that replaces OHSAS 18001. The IECB Certified ISO 45001 Lead Auditor training course helps in developing the necessary skillset to perform occupational health and safety management system (OH&S MS) audits by applying widely recognized audit principles, procedures, and methods. About This Course This training course has been developed to reflect the importance of an effective internal audit. It strengthens your knowledge and skills to plan and carry out an OH&S MS audit in compliance with the guidelines for auditing management systems provided in ISO 19011 and the certification process described in ISO/IEC 17021-1. The exercises provided for this training course are designed to help you practice the most important aspects of an OH&S MS audit: ISO 45001 requirements, auditing principles, tools and techniques used to obtain evidence, leading a team of auditors, conducting interviews with auditees, reviewing documented information, drafting nonconformity reports, and preparing the final audit report. The successful completion of the training course is followed by an exam. If you pass the exam, you gain the 'Certified ISO 45001 Internal Auditor' credential, which validates your professional capabilities and demonstrates your ability to audit an OH&S MS based on ISO 45001. Learning objectives By the end of this training course, the participants will be able to: Explain the foundational concepts and principles of an occupational health and safety management system (OH&S MS) based on ISO 45001 Interpret the ISO 45001 requirements for an OH&S MS from the perspective of an auditor Evaluate the OH&S MS conformity to ISO 45001 requirements, in accordance with the foundational audit concepts and principles Plan, conduct, and close an ISO 45001 internal audit, in accordance with ISO/IEC 17021-1 requirements, ISO 19011 guidelines, and other best practices of auditing Manage an ISO 45001 internal audit programme Educational approach This training course is participant centred and contains: Theories, best practices used in occupational health and safety management auditing Lecture sessions, which are illustrated with practical exercises based on a case study that includes role-playing and discussions Interactions, made between participants by means of questions and suggestions Quizzes, which are a simulation and preview of the certification exam Course Overview Module 1 Foundational principles and concepts of an occupational health and safety management system Module 2 ISO 45001 requirements for an OH&S MS - Clauses 4 to 10 Module 3 Foundational audit concepts and principles Module 4 Preparing for an ISO 45001 audit Module 5 Conducting an internal ISO 45001 audit Module 6 Closing an ISO 45001 audit Module 7 Managing an ISO 45001 internal audit programme Course Agenda Day 1: Introduction to OH&S MS and ISO 45001 Day 2: Audit principles and the preparation for and initiation of an audit Day 3: On-site audit activities and closing the audit, as well as the Certification exam Accreditation Assessment All candidates at official training courses are tested throughout their course with quizzes and exercises, in combination with a final exam held on the last day of the course. Both elements are a part of the overall score. For this course, the final exam constitutes a 10 question essay type exam which should be completed within 125 minutes. A passing score is achieved at 70%. Self-study candidates can purchase an exam voucher from our Store. Exam results are returned within 24 hours, with successful candidates receiving both a digital badge and a Certificate of Achievement Prerequisites The main requirements for participating in this training course are: a foundational understanding of ISO 45001 and a comprehensive knowledge of audit principles. Provided by This course is Accredited by NACSand Administered by the IECB What's Included? Refreshments & Lunch (Classroom courses only) Course Slide Deck Official Study Guides CPD Certificate The Exam Who Should Attend? Auditors interested in performing OH&S MS internal audits Managers or consultants interested in advancing their knowledge of the OH&S MS audit process Internal auditors and individuals responsible for maintaining conformity to the requirements of ISO 45001 Technical experts interested in preparing for an OH&S MS audit Expert advisors in occupational health and safety management

SAFe Advanced Scrum Master: In-House Training
By IIL Europe Ltd
SAFe® Advanced Scrum Master: In-House Training Prepare to step into a SAFe® leadership role and learn how to facilitate Agile team, program, and enterprise success by becoming a SAFe® 5 Advanced Scrum Master (SASM). This course prepares current Scrum Masters for their leadership role in facilitating Agile team, program, and enterprise success in a SAFe® implementation. Explore facilitation of cross-team interactions in support of program execution and relentless improvement. Expand the Scrum paradigm with an introduction to scalable engineering and DevOps practices, the application of Kanban to facilitate the flow of value, and supporting interactions with architects, product management, and other critical stakeholders. Learn actionable tools for building high-performing teams and explore practical ways of addressing Agile and Scrum anti-patterns in the enterprise. What you will Learn To perform the role of a SAFe® Advanced Scrum Master, you should be able to: Apply SAFe® principles to facilitation, enablement, and coaching in a multi-team environment Build a high-performing team and foster relentless improvement at scale Address Agile and Scrum anti-patterns Support the adoption of engineering practices, DevOps, and Agile architecture Learn to apply Kanban and Extreme Programming (XP) frameworks to optimize flow and improve the team's work Facilitate program planning, execution, and delivery of end-to-end systems value Support learning through participation in communities of practice and innovation cycles Exploring the Scrum Master role in the SAFe® enterprise Applying SAFe® Principles: A Scrum Master's perspective Exploring Agile and Scrum anti-patterns Facilitating program execution Improving flow with Kanban and XP Building high-performing teams Improving program performance with Inspect and Adapt

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

Business Process Modeling: In-House Training
By IIL Europe Ltd
Business Process Modeling: In-House Training This course is part of IIL's Business Analysis Certificate Program (BACP), a program designed to help prepare individuals pass the IIBA® Certification exam to become a Certified Business Analysis Professional (CBAP®). Learn more at www.iil.com/bacp A process model is a description of a process in terms of its steps or actions, the data flowing between them and participants in the process, machines, systems, and organizations involved. Modeling is a critical business analysis skill. It applies graphical and text communication techniques to describe the actions, objects, and relationships acted upon in the process and the steps that act upon them. This course teaches the technique of process modeling and ties together the core methods of process, behavior, and data modeling to enable business analysts to fully describe business processes in levels of detail from multiple perspectives. What you will Learn Upon completion, participants will be able to: Identify business processes and their components Work with UML diagrams Use process modeling in business diagramming Diagram and model business processes Foundation Concepts The role of the business analyst The IIBA® BABOK® Knowledge Areas Business Process Modeling (BPM) and the business analyst A practical approach to business process modeling The Context for Modeling Business Processes Overview of context for business process modeling Analyzing stakeholder information Modeling best practices Critical inputs for BPM: Business Rules Critical inputs for BPM: Context Diagrams Data Models Overview of data modeling Entity relationship diagrams Object-oriented approach Class diagrams Other data models Process Models - Part I (Non-UML) Overview of process modeling Data flow diagrams Workflow diagrams Flowcharts Process Models - Part II (UML) Overview of UML Process Models UML Activity Diagrams UML Sequence Diagrams Usage Models - Part I (Non-UML) Overview of usage modeling Prototyping options Static prototyping and storyboards Dynamic prototyping User Interface Design and user stories Usage Models - Part II (UML Use Cases) Overview of Use Cases Use Case diagrams Use Case descriptions Use Cases and the product life cycle Integrating the Models Overview of integrating the models General analysis best practices Specific analysis techniques summary Best practices for transition to design Summary and Next Steps What did we learn and how can we implement this in our work environments?

ISO 37001 Internal Auditor
By Training Centre
The IECB Certified ISO 37001 Internal Auditor training course provides the necessary knowledge and skills that enable you to perform anti-bribery management system (ABMS) audits by applying widely recognized audit principles, procedures, and techniques. About This Course Many organizations seek competent auditors to determine whether the policies and anti-bribery controls, implemented as part of an ISO 37001-based ABMS, are effective. This training course aims to help you complete these tasks successfully and intends to reflect the importance of effective ABMS audits. Additionally, it aims to strengthen your knowledge and skills to plan and carry out ABMS audits in compliance with the guidelines for auditing management systems provided in ISO 19011 and the certification process described in ISO/IEC 17021-1. The exercises, quizzes, and case studies provided are designed to help you practice the most important aspects of an ABMS audit: ISO 37001 requirements, auditing principles, tools and techniques used to obtain evidence, leading a team of auditors, conducting interviews with auditee, reviewing documented information, drafting nonconformity reports, and preparing the final audit report. The successful completion of the training course is followed by an exam. If you pass the exam, you gain the 'Certified ISO 37001 Lead Auditor' credential which validates your professional capabilities and demonstrates your ability to audit an ABMS based on ISO 37001. Learning objectives By the end of this training course, the participants will be able to: Explain the foundational concepts and principles of an anti-bribery management system (ABMS) based on ISO 37001 Interpret the ISO 37001 requirements for an ABMS from the perspective of an auditor Evaluate the ABMS conformity to ISO 37001 requirements, in accordance with the foundational audit concepts and principles Plan, conduct, and close an ISO 37001 compliance audit, in accordance with ISO/IEC 17021-1 requirements, ISO 19011 guidelines, and other best practices of auditing Manage an ISO 37001 Internal audit programme Educational approach This training course is participant centred and contains: Theories, approaches, and best practices used in anti-bribery management system auditing Lecture sessions, which are illustrated with case studies inspired by real events and practical exercises based on a case study that includes role-playing and discussions Interaction between participants by means of questions and suggestions Course Overview Module 1 Foundational principles and concepts of an anti-bribery management system Module 2 Anti-bribery management system requirements Module 3 Foundational audit concepts and principles Module 4 Preparing an ISO 37001 audit Module 5 Conducting an ISO 37001 audit Module 6 Closing an ISO 37001 audit Module 7 Managing an ISO 37001 Internal audit programme Course Agenda Day 1: Introduction to the anti-bribery management system (ABMS) and ISO 37001 Day 2: Audit principles and the preparation for and initiation of an audit Day 3: On-site audit activities, Closing of the Audit and the Certification exam Accreditation Assessment All candidates at official training courses are tested throughout their course with quizzes and exercises, in combination with a final exam held on the last day of the course. Both elements are a part of the overall score. For this course, the final exam constitutes a 10 question essay type exam which should be completed within 125 minutes. A passing score is achieved at 70%. Self-study candidates can purchase an exam voucher from our Store. Exam results are returned within 24 hours, with successful candidates receiving both a digital badge and a Certificate of Achievement Prerequisites A foundational understanding of ISO 37001 requirements for an ABMS and a comprehensive knowledge of audit principles. What's Included? Refreshments & Lunch (Classroom courses only) Course Slide Deck Official Study Guides CPD Certificate The Exam Provided by This course is Accredited by NACS and Administered by the IECB Who Should Attend? The ISO 37001 Internal Auditor training course is intended for: Auditors seeking to perform and lead ABMS audits Managers or consultants seeking to master the ABMS audit process Individuals responsible for maintaining conformity to ISO 37001 requirements in an organization Technical experts seeking to prepare for an ABMS audit Expert advisors in anti-bribery management

Carrying out successful property valuations/marketing appraisals
By Mark Bentley PPNAEA (Mark Bentley Ltd)
By the end of the course you will have picked up numerous tips and guidance and advice to help you carry out great property valuations/market appraisal's which will help you improve you conversion rate and win more clients.

Managing Remote and Virtual Teams
By Nexus Human
Duration 2 Days 12 CPD hours Overview Effectively manage team dynamics in remote and virtual teams Leverage communication technologies to the benefit of your remote and virtual teams Identify the specific skills required for managing remote and virtual teams Evaluate the impact of culture and language on your team?s performance The business model of managing remote and hybrid teams is constantly evolving. Managing remote teams?a rarity just a few years ago?is now a common occurrence. Working virtually offers unique advantages and challenges. But how do you best leverage these benefits while overcoming impediments? This workshop will teach you to adjust your management style to successfully improve communication, foster connections, increase productivity, and develop remote and virtual teams. Focused on practical skills, this workshop includes activities to apply these techniques and drive results. Defining the Characteristics of the Remote and Hybrid Workforce Understanding the remote vs. hybrid workplace Managing relationships, communication, and tasks Meeting your needs and your team?s needs Management Requirements for Remote Leadership Moving from reactive to proactive Understanding team member?s unique situations Keeping everyone informed Innovating with virtual teams Managing work outputs Overseeing separated team members Defining and building relationships with stakeholders Building and Strengthening Team Dynamics Creating team identity Forming remote and virtual teams Managing the storming process Getting to norming and performing Creating and governing with ground rules Tracking team performance Setting expectations and providing feedback Making Technology Work for You Communicating with and coordinating your team Avoiding the technology trap Developing effective communication across various mediums Choosing the right technology platform The Impact of Culture and Separation Recognizing cultural characteristics and differences Building cultural knowledge Managing across time zones Respecting non-working time

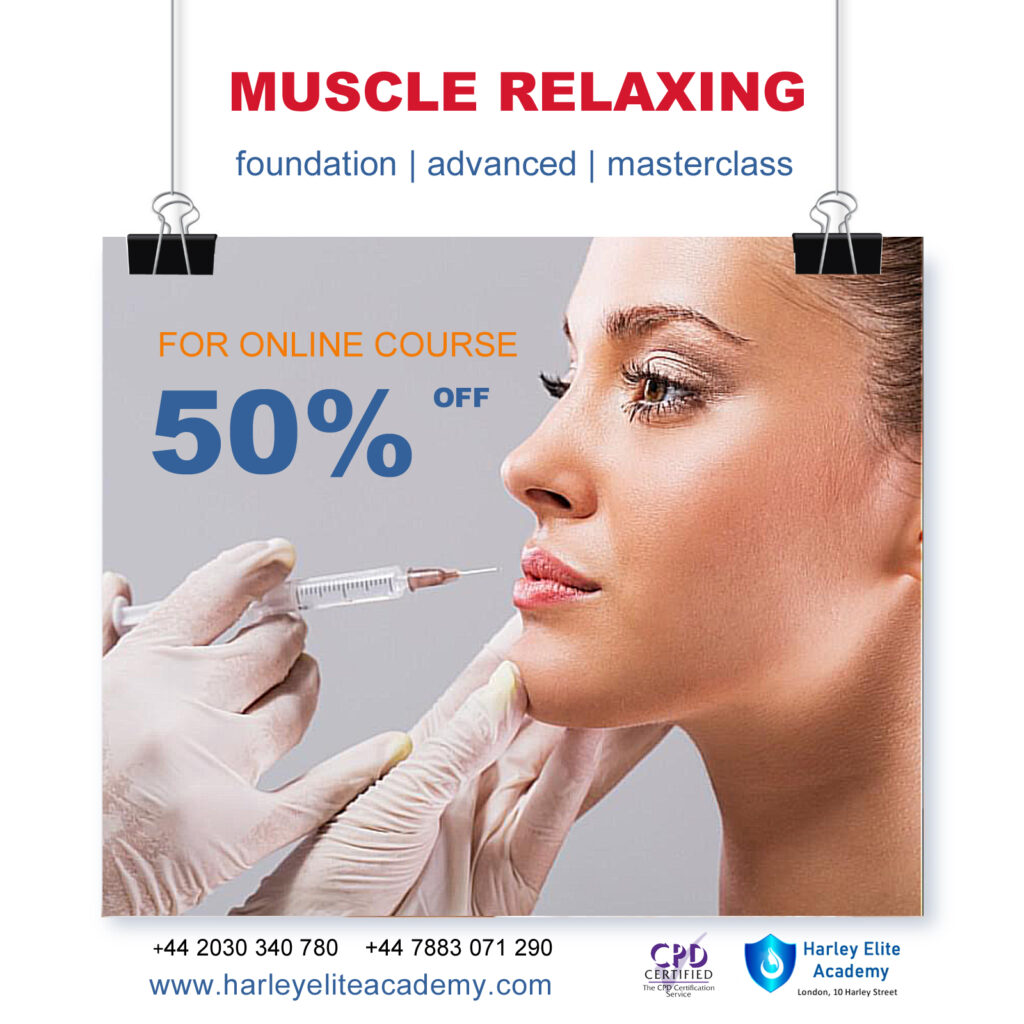
MUSCLE RELAXING | BOTOX®
By Harley Elite Academy (HeLa)
Foundation • Advanced • Masterclass 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. Additional information ATTENDANCE ONLINE (theory), IN-CLINIC (Practice) COURSE LEVEL BEGINNER | Foundation Course, INTERMEDIATE | Advanced Course, EXPERT | Masterclass Course
Rapid Prototyping with Axure
By Bunnyfoot
This one-day course introduces the field of user experience and provides an excellent entry point to our other specialised training courses. UX processes and practices have become a central component of product design, service design and web design.

Film and Game Design Training Classes
By ATL Autocad Training London
Who is this course for? Film and Game Design Training Classes is tailored for individuals passionate about 3D for Games. Ideal for those in London seeking specialized skills for lucrative job opportunities in the gaming industry. Software we teach: 3ds max or Maya, Vray, After effects and Photoshop. Check our Website Duration: 40 hours. 1-on-1 Training. When can I book: 9 am - 4 pm (Choose your preferred day and time once a week). Monday to Saturday: 9 am - 7 pm (Flexible timing with advance booking). Course Title: Film & Game Design Training Classes Option A - 40-Hour Program: Option A offers comprehensive training for aspiring film and game designers, covering vital industry software and skills. Module 1: 3ds Max and Advanced Animation (20 hours) - Introduction to 3ds Max: Interface overview. - Basic 3D Modeling: Creating simple 3D objects. - Advanced 3D Modeling: Complex modeling techniques. - Texturing and Materials: Applying textures and materials. - Lighting and Rendering: Scene lighting and rendering setup. - Character Animation: Rigging and animating characters. - Advanced Animation Techniques: Keyframes, motion paths, and more. - Scene Composition: Assembling complex scenes. Module 2: Vray (6 hours) - Vray Introduction: Understanding Vray renderer. - Lighting with Vray: Creating realistic lighting setups. - Material Creation: Crafting materials for realistic surfaces. - Rendering with Vray: Optimization and execution. Module 3: Photoshop (6 hours) - Photoshop Basics: Navigating the interface. - Image Editing: Crop, resize, and enhance. - Layer Management: Working with layers. - Text and Typography: Adding and manipulating text. - Photo Manipulation: Advanced image techniques. - Creating Visual Assets: Designing textures and graphics. Module 4: After Effects: Video and Sound Editing (8 hours) - Introduction to After Effects: Interface overview. - Video Editing: Cut, trim, and arrange video clips. - Transitions and Effects: Apply visual effects and transitions. - Sound Editing: Add and edit audio tracks. - Motion Graphics: Create motion graphics and titles. - Exporting and Rendering: Prepare projects for final output. Film & Game Design Training Course Information Are you ready to explore our Training Course for Film & Game Designers? Here's a comprehensive overview to guide you through: When Can I Book This Training Course? Personalize your training with our flexible 1-on-1 sessions. Tailor your schedule by pre-booking your preferred hours. Available Monday to Saturday, 9 a.m. to 7 p.m. For phone bookings, call 02077202581. Training Duration The course spans 40 hours, allowing flexibility for your ideal schedule. Training Method Experience 1-on-1 training, in-person Face to Face or Live Online. Expect personalized attention, tailored content, flexible learning, and individual support. Opt for Live Online 1-on-1 sessions via Zoom for convenience. Enroll Today Ready to start your exciting journey? Click the link below to enroll in our 1-on-1 Course. Film & Game Design Training Overview In our comprehensive training program for film and game designers, refine your skills using industry-leading software tools. This prepares you to bring your creative visions to life. Option A: 3ds Max and Advanced Animation (20 hours) Vray (6 hours) Photoshop (6 hours) Aftereffects: Video and Sound Editing (8 hours) Option B: Maya and Advanced Animation (20 hours) Vray (6 hours) Photoshop (6 hours) Aftereffects: Video and Sound Editing (8 hours) Both options offer flexibility for Mac and Windows operating systems, ensuring accessibility for all learners. Key Benefits Price Assurance: Exceptional value for your film and game design career investment. One-on-One Training: Customized learning for your unique style. Flexible Scheduling: Choose your training time, available Monday to Sunday, 9 am to 8 pm. Lifetime Email and Phone Support: Ongoing assistance beyond training for your career growth. Computer Configuration Assistance: Guidance for seamless software installation. Referral Benefits: Special discounts for referrals and savings on group training. Embark on a transformative journey and unlock your potential in the thrilling fields of film and game design!
