- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Microsoft Access Advanced - In-company / Bespoke
By Microsoft Office Training
Course Objectives At the end of this course you will be able to: Do advance Table design Do advance Query design and Action Querys Do advance Form design with the use of macros and buttons Export and import data to and from different sources. 1 year email support service Take a look at the consistent excellent feedback from our corporate clients visiting our site ms-officetraining co uk With more than 20 years experience, we deliver courses on all levels of the Desktop version of Microsoft Office and Office 365; ranging from Beginner, Intermediate, Advanced to the VBA level. Our trainers are Microsoft certified professionals with a proven track record with several years experience in delivering public, one to one, tailored and bespoke courses. Our competitive rates start from £550.00 per day of training Tailored training courses: You can choose to run the course exactly as they are outlined by us or we can customise it so that it meets your specific needs. A tailored or bespoke course will follow the standard outline but may be adapted to your specific organisational needs. Advanced Table Design Advanced Field Properties Table Properties Advanced Query Design Advanced Naming Conventions Join Tables in Queries Manage Query Joins Use Self-Joins in Queries Summarise Data in Queries Parameter Queries Action Queries Crosstab Queries Advanced Form Design Create Subforms and Linked Forms Form Controls Command Buttons Form Properties Interface, Start-Up and Navigations Forms Working with Macros Create Single Macros Run Macros Work with Sub Macros Use Conditional Macros Run Macros from Buttons Assign Macros to Events Extending Data Reach Import Data Export Data Work with Linked Tables Managing Databases Object Dependencies Database Documenter Performance Analyzers Regular Management of a Database Access Database Security Who is this course for? Who is this course for? The course is aimed at all users who would like to obtain the necessary skills to create advanced table, query, form and reports as well as to automate tasks with the use of macros. Career path Career path Microsoft Office know-how can instantly increase your job prospects as well as your salary. 80 percent of job openings require spreadsheet and word-processing software skills

Geometry of Ancient Egypt with Adam Tetlow
By Sacred Art of Geometry
Join us on a journey through the geometric arts of Ancient Egypt ... The profound relationship between qualitative geometry and the forms of architecture and sculpture is no where better expressed than in the ancient egyptian tradition. During this course we will be drawing inspiration from the work of R.A. Schwaller de Lubitcz, John Michell and others. The philosophical implications of the golden section in Egyptian art will be covered. The Pyramids: the pyramids and earth measure - we will draw and model the great pyramid of Giza to scale.

Racing Simulator Driving Sessions
By Rally Navigation Training Services
Racing Simulator coaching session.

Maximizing Academic Success: How to Use a Free Assignment Sample in UK
By david hude
This article explores the advantages of using a Free Assignment Sample in UK to improve academic performance. It highlights how New Assignment Help provides valuable resources tailored to UK academic standards, assisting students in creating well-structured, high-quality assignments.

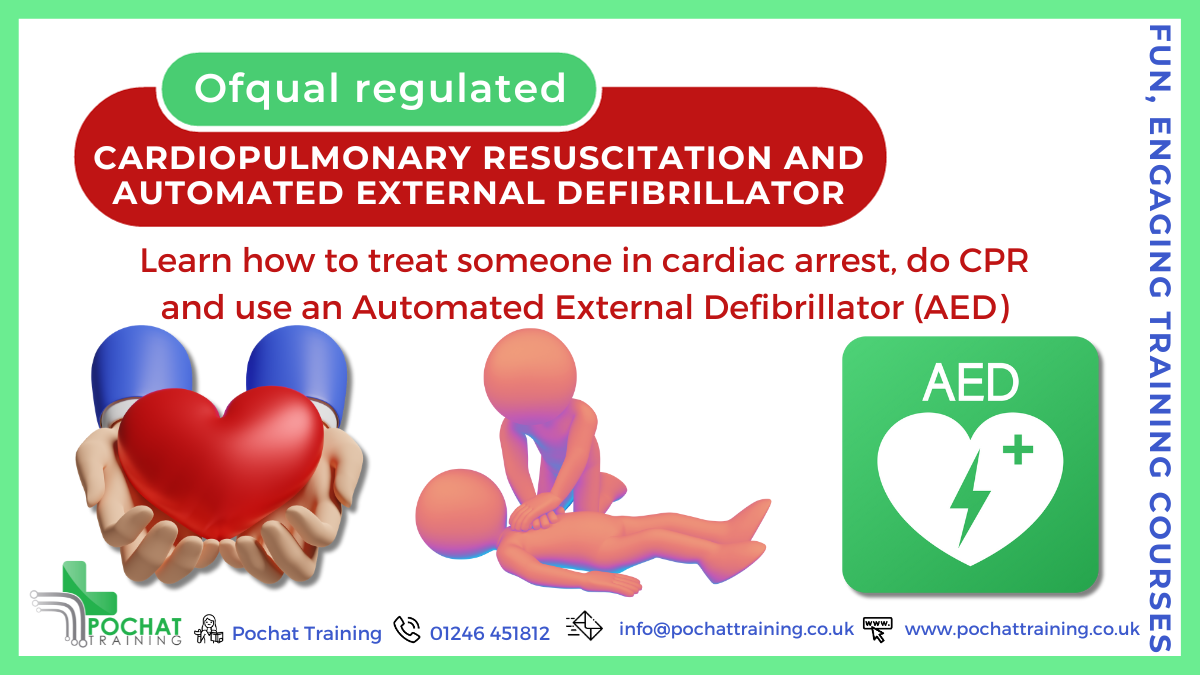
QA Level 2 Award In Cardiopulmonary Resuscitation And Automated External Defibrillation (RQF) Full day course Gives learners the knowledge and skills to give CPR and use an AED safely Covers maintenance of AEDs (Automated External Defibrillators) Course Contents: The principles underlining basic life support The principles underlining resuscitation Different types of cardiac arrest Recovery Position CPR Choking Safe Use of an AED (Automated External Defibrillator) AED maintenance Benefits of this course: In the UK there are over 30,000 cardiac arrests a year outside of hospital But only less than one in ten survive to be discharged from hospital When someone has had a cardiac arrest, every minute makes a huge difference You can help save people's life by starting CPR as soon as possible, and use an AED (Automated External Defibrillator) to restart their heart This course helps to give you the skills and confidence to save people's lives, and make a huge difference not just to them, but also to their families and loved ones Accredited, Ofqual regulated qualification: Our Cardiopulmonary Resuscitation and Automated External Defibrillation course is a nationally recognised, Ofqual regulated qualification accredited by Qualsafe Awards. This means that you can be rest assured that your CPD & AED Certificate is a very good way to make sure you and your employees are trained in First Aid. And of course, all of our training courses are run in a fun and engaging, interactive and varied way, ensuring your employees have the skills and knowledge they need! The Ofqual Register number for this course is 603/2654/2 This page is here if you'd like us to run this course for you and your people, at our venue or yours (within 45 minute drive from Chesterfield, Derbyshire). If you'd like us to run this course for you and you're further away, please contact us direct for a quote. If, instead, you're interested in an open/public course, please go here.
Microsoft Excel Macros & VBA - In-company
By Microsoft Office Training
Course Objectives At the end of this course you will be able to: Record and edit a Macro Assign macros to keyboard shortcuts, Quick Access Toolbar, Buttons and Shape objects. Use the Visual Basic Editor; change the properties of an object; add a module to a project; write the code for a procedure and then run it; and use the Object Browser to search procedures Manipulate data by declaring variables of different data types; combine data by using expressions; use functions to accept input and display output; and declare variables and procedures with the appropriate scope Use decision structures to create procedures that make decisions; and use loop structures to perform repetitive tasks Create an error handling routine in case things go wrong with VBA code ' 1 year email support service Take a closer look at the consistent excellent feedback from our growing corporate clients visiting our site ms-officetraining co uk Customer Feedback Best Training Ever! Just finished a bespoke 1-1 training course in Excel Advanced, Macros & VBA. Pedro is an excellent trainer, imparting his skills and knowledge in the best way - appropriately to audience skills, knowledge and ability. Pedro is always approachable, encouraging and supportive, giving delegates the optimum learning environment. I would not hesitate to recommend Pedro as a trainer, whatever your level of ability. Amanda Morris - Treasury & Systems Accountant at Reall - Real Equity for All The trainer was very knowledgeable, kept everyone involved and was enthusiastic. A great experience.. Simon Harper - Lloyd's of London My learning experience was awesome. Perdinand Reagan - Subsea7 Very nice and relaxed approach to teaching. Was definitely a good learning experience. Jerome Pupe - S5 Agency World The Trainer – Very positive + patient + helpful + thorough Agnes Souza - Direct Wines ' With more than 20 years experience, we deliver courses on all levels of the Desktop version of Microsoft Office and Office 365; ranging from Beginner, Intermediate, Advanced to the VBA level. Our trainers are Microsoft certified professionals with a proven track record with several years experience in delivering public, one to one, tailored and bespoke courses. Our competitive rates start from £550.00 per day of training Tailored training courses: You can choose to run the course exactly as they are outlined by us or we can customise it so that it meets your specific needs. A tailored or bespoke course will follow the standard outline but may be adapted to your specific organisational needs. Introduction to Macros and VBA Introducing Visual Basic for Applications Recording a Macro Naming conventions for Macro Procedures Running a Macro Absolute or Relative Cell Referencing Saving and Opening Files with Macros Making macros always available Adding Macros to Keyboard Shortcuts, Quick Access Toolbar, Buttons and Shapes Editing a Macro in the Visual Basic Editor Understanding the Development Environment Customising the Editor Tips for General Typing in VBA Using Visual Basic Help Working with Procedures Program Design Understanding Modules Naming Rules Creating a Module Understanding Procedures Programming Macro Concepts Creating a Subroutine Creating a Function Understanding Arguments Exiting Procedures Calling Procedures Objects, Properties, Methods and Events Understanding Objects Properties, Methods, and Events Navigating the Object Hierarchy Understanding Collections Accessing a Member of a Collection Understanding Hierarchy Using the Object Browser Using the With Statement Working with Properties Working with Methods Creating an Event Procedure Reserved Macro Names Using Expressions, Variables and Intrinsic Functions Understanding Expressions and Statements Declaring Variables Understanding Data Types Determining the Value of Variables Working with Variable Scope Using Built-in VBA Functions Understanding Constants Using Message Boxes Controlling the Answer to a Message Box Using Input Boxes Declaring and Using Object Variables Controlling Program Execution Understanding Control-of-Flow Structures Using the If...End If Decision Structures Nested If Statements Using the Select Case ... End Select Structure Using the Do ... Loop Structure Using the For ... Next Structure Using the For Each ... Next Structure Guidelines for Use of Control-of-Flow Structures Debugging and Handling Errors Understanding Errors Using Debugging Tools Setting Breakpoints and Using Break Mode Stepping Through Code Trapping Errors with the On Error Statement Understanding the Err Object Working with Inline Error Handling Writing an Error-Handling Routine Working with Forms and Controls Understanding UserForms Creating a Form Displaying and Removing a Form Aligning and Sizing Controls Using the Toolbox Working with a Form's Properties, Methods, and Events Working with Form and Control Properties, Methods and Event Setting the Tab Order Populating a Control Who is this course for? Who is this course for? This course is designed to give proficiency in the Visual Basic Editor (VBE), predominantly making use of Excel objects, understanding Object's Properties, Events and Methods, basic VBA Object oriented programming, event handling, control structures, and debugging tools. Requirements Requirements Preferably, delegates should have attended the Excel Advanced course. Certificates Certificates Certificate of completion Digital certificate - Included

Introduction to Exploration and Production for New Engineers and Non-Technical Professionals in Oil & Gas (2 Days)
By EnergyEdge - Training for a Sustainable Energy Future
Enhance your knowledge of exploration and production in oil & gas with EnergyEdge's course. Ideal for new engineers and non-technical professionals.

Introduction to Exploration and Production for New Engineers and Non-Technical Professionals in Oil & Gas (2 Days)
By EnergyEdge - Training for a Sustainable Energy Future
Enhance your knowledge of exploration and production in oil & gas with EnergyEdge's course. Ideal for new engineers and non-technical professionals.

A System Approach to Good Pharmacovigilance Practice
By Research Quality Association
Course Information In today's evolving landscape, pharmacovigilance (PV) systems face ongoing challenges due to global, national, and company-specific events. This course focuses on developing personnel equipped to navigate these complexities and improve the safe use of medicinal products. We emphasise continuous global thinking, communication, and strategic planning, ensuring adaptability across various levels. The course explores maintaining PV system functionality while adhering to regulatory requirements. Participants will apply European regulatory standards to enhance and sustain PV system effectiveness, contributing to improvement initiatives and ensuring operational integrity. The course covers PV system intricacies, regulatory compliance, and a 'systems approach' for auditors, quality assurance personnel, and PV practitioners. Through presentations and workshops, attendees gain insights into implementing and maintaining an effective PV system. Who should attend? Auditors Pharmacovigilance Quality System Managers Pharmacovigilance scientists The QPPV. Course benefits Throughout the course delegates will explore application of the legal requirements for the PV system and quality system and how to assure these systems. They will demonstrate their ability to contribute to: A systematic investigation of the pharmacovigilance system and its quality system Examination of how the pharmacovigilance system and quality system interact to achieve compliance. The risk-based approach to auditing the PV system and quality system The maintenance of 'inspection readiness' Explore how to investigate the complex PV system Discussions about how to monitor and maintain the PV system and assure compliance. Course Objectives Clarify what has to be done: Explore application of the legal requirements. Explore how to do what has to be done: Adopt a systemic approach to systematically investigate or implement and maintain the PV system and quality system Examine how a compliant PV system and a compliant quality system interact to achieve compliance with regulatory requirements for PV Explore how to investigate the complexity of the PV system. Discus how to identify what is missing or what needs to be improved: Discuss how to monitor and maintain the PV system and assure compliance. This course will assist delegates with: An understanding of key system principles, A practical approach to implementing, maintaining and monitoring the PV system and its quality system A procedure to share expertise to increase efficiency and confidence. This course is structured to encourage delegates to: Discuss and develop ideas - Share knowledge and experiences - Solve specific problems. By the end of the course delegates will be able to: Understand better the pharmacovigilance system, its quality system and how the components interact to achieve the objectives of pharmacovigilance Investigate, and analyse the pharmacovigilance system and to identify what is missing and what needs to be improved. Tutors Tutors will be comprised of (click the photos for biographies): Jana Hyankova Head of PV Department, IVIGEE Services a.s. Programme Please note timings may be subject to alteration. Day 1 08:30 Welcome, registration, course objectives and introduction to work groups Housekeeping notices, meet other delegates, explore how to work in your work group, course objectives. Clarify the definition and objectives of Pharmacovigilance. 09:30 The Regulatory Framework for Pharmacovigilance Identify the relevant regulations and directives. Explore GVP guidance, structure of the modules and standard format of each module. 10:00 The Pharmacovigilance System Exploration of how to organise what has to be done, communications. Exploration of the structures and processes for pharmacovigilance. 10:30 Break 11:00 Workshop 1 and Feedback Exploring an organisational model of the pharmaceutical company- cooperation between PV and other stakeholders. 12:00 The Quality System for pharmacovigilance Exploration of the structures, processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 13:00 Lunch 14:00 Workshop 2 and Feedback The quality system puzzle. Explore the organisation of the PV quality system and how it interacts with the PV system. 14:30 The Quality System for pharmacovigilance Exploration of the structures processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 15:00 Description of PV System 15:30 Break 15:30 Workshop 3 and Feedback The quality system puzzle Explore the organisation of the PV quality system and how it interacts with the PV system. 16:00 The Pharmacovigilance Safety Master File Construction of the Pharmacovigilance System Master File and its purpose. 17:00 Workshop 3 and Feedback Description of PV System. 18:00 End of Day Day 2 08:30 Drug Safety in the Clinical Trial Environment - Part 1 Information flow and responsibilities of the sponsor. 09:30 Workshop 4 and Feedback Drug Safety in the Clinical Trial environment: Information flow and responsibilities of the sponsor. 10:30 Break 11:00 Drug Safety in the Clinical Trial Environment - Part 2 Information flow and responsibilities of the sponsor. 12:00 Lunch 13:00 Workshop 5 and Feedback Drug Safety in the clinical trial environment: Information flow and responsibilities of the sponsor. 13:30 Processing of Safety Data Exploration of safety data processing, verification, validation, follow up, formatting and collation, reporting requirements, quality and data management. 15:00 Break 15:30 EudraVigilance Exploration of how EudraVigilance supports the PV system. 16:15 Signal Detection and Evaluation/Risk Benefit Assessment: Pharmacovigilance Risk Assessment Committee (PRAC): What is a signal? What are the regulatory requirements? How is signal detection and evaluation conducted? Qualitative and quantitative methods of signal detection. Risk benefit assessment. 17:00 Risk Management Plans A cornerstone of Pharmacovigilance safety communications, direct healthcare professional communication 18:00 End of Day Day 3 08:30 The Pharmacovigilance Risk Assessment Committee (PRAC) Exploration of how good practice is achieved. Composition, role and responsibilities. Examples of referrals. 09:15 Development Safety Update Reports (DSURs): Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:30 Break 11:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 12:00 Workshop 6 and Feedback To explore the compilation and submission of the PSUR. 13:00 Lunch 13:30 Role of the QPPV Exploration of the legal responsibilities of the QPPV and the MAH. 14:30 Break 15:00 Workshop 7 and Feedback To explore the challenges faced by the QPPV. 15:30 End of course Extra Information Face-to-Face Course Course material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device< Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD Points 23 Points Development Level Develop

Search By Location
- uk Courses in London
- uk Courses in Birmingham
- uk Courses in Glasgow
- uk Courses in Liverpool
- uk Courses in Bristol
- uk Courses in Manchester
- uk Courses in Sheffield
- uk Courses in Leeds
- uk Courses in Edinburgh
- uk Courses in Leicester
- uk Courses in Coventry
- uk Courses in Bradford
- uk Courses in Cardiff
- uk Courses in Belfast
- uk Courses in Nottingham
