- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
693 Courses
Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

Good Laboratory Practice for Study Directors, Principal Investigators, Study Staff and Management
By Research Quality Association
Course Information Embark on our GLP course offering extensive guidance and pragmatic support tailored for individuals serving as Study Directors or Principal Investigators overseeing non-clinical safety studies on pharmaceuticals, agricultural, and industrial chemicals within the realm of Good Laboratory Practice (GLP). This comprehensive programme extends its benefits to study staff and management operating in GLP-compliant environments. The course extensively covers the current OECD GLP Principles and UK GLP legislation, while also referencing international standards, regulations, and guidelines pertinent to the field. Benefits of this course: Practical help and guidance on the interpretation and application of GLP An opportunity to update your knowledge of GLP with the current interpretation of requirements Access to an experienced panel of speakers Information on how other organisations address GLP issues An opportunity to improve your understanding of the GLP requirements as they are applied in different situations. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of GLP Learn from the experience of others. Tutors Tutors will be comprised of (click the photos for biographies): Tim Stiles Consultant, Qualogy Ltd Tony Woodall Head of Quality Assurance, Alderley Analytical Gill Armour Study Monitor Team Leader, AstraZeneca Jane Elliston Senior Quality Assurance Auditor, Battelle UK Vanessa Grant -, - Jeanet Logsted CEO, Scantox Programme Please note timings may be subject to alteration. Day 1 09:00 Registration 09:15 Welcome and Introductions 09:35 Development of Good Laboratory Practice A review of the history of GLP, its current scope and application, with a synopsis of current European and international standards. 10:05 Roles and Responsibilities The responsibilities of study director, test facility, management and study staff in the conduct of a GLP study. 10:45 Break 11:00 The Roles and Responsibilities of the Study Director and Test Facility Management The role of the study director in the management and control of a study, as defined by GLP, and management's roles are explored. 11:45 Multi-site Studies What is a multi-site study and when should such concepts be applied on a study. The role of the study director and principal investigator in the planning, conduct and reporting of multi-site study are explored. 12:30 Study Plan (Protocols) GLP requirements for the preparation of a study plan, content, authorisation, amendments and deviations are discussed. 13:00 Lunch 13:45 Workshop 1 - The Study Plan Some practical problems with study plans and amendments explored. 14:45 Workshop 1 - Feedback 15:00 Standard Operating Procedures The control, content and authorisation of SOPs and the principles behind the practice. 15:30 Break 15:45 Workshop 2 - Practical Study Conduct Problems Dealing with practical problems encountered during the conduct of studies. 16:40 Workshop 2 - Feedback 17:15 Close of Day Day 2 09:00 Questions and Answers Discussion of issues raised by course delegates. 09:20 Quality Assurance The interactions between QA, management, study director and principal Investigator are discussed as is QAs role when conducting a multi-site study. 10:00 The Final Report The content of the final report and the role of those involved in its preparation and approval. Specific reporting requirements when conducting a multi-site study are also explained. 10:30 Break 10:45 Workshop 3 - Final Report Problems Practical problems of report preparation including compliance statements. 11:30 Workshop 3 - Feedback 12:00 Management of Raw Data and Records A view on how records and materials are managed and archived in compliance with GLP. 12:45 Lunch 13:30 Workshop 4 - Data and Sample Management Issues Dealing with data and sample management issues. 14:15 Workshop 4 - Feedback 14:45 Regulatory Inspection Government monitoring for compliance with Good Laboratory Practice. 15:15 Panel Session This panel session will address any outstanding issues raised by delegates. 15:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

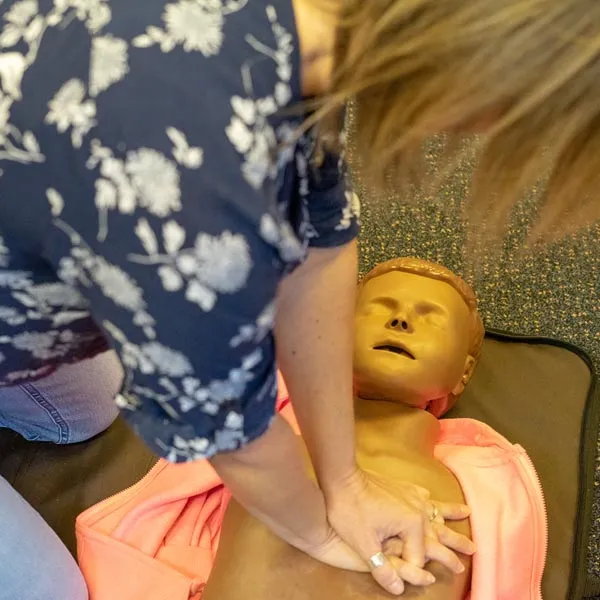
First Aid at Work (Re-Qual) - Level 3 Award
By Immerse Medical
Our 2 day course will enable students who have successfully completed a Level 3 Award in First Aid at Work (QCF or RQF) to re-certify. This first aid at work course is ideal for organisations whose needs assessment has identified a requirement for additional first aid training, such as having employees with a disability or a medical condition. In addition to the topics covered on an emergency first aid at work course, this course covers treatment for a variety of injuries and medical conditions. For more information click on the tabs below, or get in touch, we’d be more than happy to answer any queries. At Immerse Training we pride ourselves on offering First Aid and Pre-Hospital Care Training that meets your specific needs. All our courses meet the requirements of the relevant awarding body. On top of that, we are more than happy to create bespoke elements that tailor each programme to suit your first aid or care responsibilities. Qualification Information This qualification and learning outcomes are based on the recommendations of: The Resuscitation Council (UK) Skills for Health Assessment Principles for First Aid Qualifications Course Content Following this course students will be able to Understand the role and responsibilities of a first aider. Be able to administer first aid to a casualty with injuries to bones, muscles and joints. Assess an incident. Be able to administer first aid to a casualty with suspected head and spinal injuries. Manage an unresponsive casualty who is breathing normally. Be able to administer first aid to a casualty with suspected chest injuries. Manage an unresponsive casualty who is not breathing normally. Be able to administer first aid to a casualty with burns and scalds. Be able to recognise and assist a casualty who is choking. Be able to administer first aid to a casualty with an eye injury. Be able to manage a casualty with external bleeding. Be able to administer first aid to a casualty with sudden poisoning. Be able to manage a casualty who is in shock. Be able to administer first aid to a casualty with anaphylaxis. Be able to manage a casualty with a minor injury. Be able to provide first aid to a casualty with suspected major illness. Be able to conduct a secondary survey. Who should attend? This qualification is for people who deal with first aid at work. Enabling them to be workplace first aiders under the Health and Safety (First Aid) Regulations 1981. This qualification is also for people who have a specific responsibility at work, or in voluntary and community activities. This will allow them to provide basic first aid to people in a range of situations. Pre-requisites Students must be at least 14 years old on the first day of training. Students must be in possession of an in date First Aid at Work Certificate. Assessment and Certifications Assessment of this course is continuous and includes two theory/multiple choice question papers. Successful students will receive an Immerse Training Certificate, which is valid for three years. This certificate will be issued by Qualsafe, the awarding body for Immerse Training. Additional Information Completion of the Level 3 Award in First Aid at Work includes 3 credits at Level 3 of the Regulated Qualifications Framework (RQF). Workplace First Aid Courses First Aid courses for employers and employees. Our workplace courses are fully accredited, registered and meet Health and Safety Executive (HSE) guidelines. From 1 day Emergency First Aid at Work (previously appointed person) to 3 day First Aid at Work courses. We specialise in on-site courses at your workplace, tailored to the specific risks associated with your business. All courses can be delivered at our training centre in Poole, Dorset or we can deliver on-site across Bournemouth, Poole, Dorset, Hampshire and the South of England.
Leading mental health for supervisors, team leaders and managers is about leading your team and mental health first aiders to a healthy productive way, increasing respect, getting a mutual understanding for todays and tomorrows workforce.

DMI PRO-Certified Digital Marketing professional
By London School of Science and Technology
Boost your career and stay relevant with the world’s most recognized Digital Marketing diploma. Continuously updated content means you’ll get cutting-edge digital marketing and soft skills, always. Course Overview Boost your career and stay relevant with the world’s most recognized Digital Marketing diploma. Continuously updated content means you’ll get cutting-edge digital marketing and soft skills, always. Learn Google Ads, social media marketing, SEO and much more. Become a certified digital marketing professional with DMI. Program learning outcomes and content: What Will I Learn? You will be armed with the know-how, the experience and the insights to be able to work and speak with authority in this fast-paced industry. In short, you’ll be a skilled digital marketer, capable of building digital marketing strategies from scratch. Who is the DMI Pro for? • Traditional Marketers or Marketing Executives who want to play a bigger, sharper game • Marketing Managers and Senior Management • Graduates with no plans to wait around • Small Business Owners who want to grow • Career Changers • Entrepreneurs who want to be more entrepreneurial • Whoever needs to create and apply a digital marketing strategy for their organisation Course Content: Introduction to Digital Marketing: What’s it all about? How do you reach customers? How can traditional and digital media work together? What’s the difference between inbound and outbound marketing? This module takes you through the basics, keeping things clear and actionable: • Principles of Digital Marketing • Digital Research • Developing Objectives • Cultural Research • Connecting with the Customer Social Media Marketing: Bring your brand story to life on all the right platforms. Learn how to grow and engage a community around your offering. Define your audience and give them more to care about. There is so much to ‘like’. • Key Social Platforms for Digital Marketing • Growing and Engaging an Audience • Developing Data-Driven Audience and Campaign Insights • Setting up a Social Media Experience for a Business • Creating and Optimising Social Media Campaigns Paid Search (PPC) using Google Ads: Develop Pay-Per Click skills using Google Ads, bid auctions and build your targeting strategy. Optimise campaigns, track conversions, and measure your ROI. • Fundamentals of Paid Search • Search Campaign Management • Paid Search Campaign Measurement • Paid Search Campaign Creation with Google Ads Email Marketing: Your email list is one of your most powerful assets. Learn how to manage and segment your data, test headlines and maximise open rates and ROI. We also cover marketing automation and the importance of data management regulations. • Email Marketing Fundamentals • Email Design • Testing and Optimising an Email Campaign • Tools and Strategy • Creating an Effective Email Campaign • Marketing Automation Analytics with Google Analytics: This module helps you unleash the capability of your data. Discover what your customer wants, likes, needs and does. Understand how they use your website, set up goals and monitor conversions. • Web Analytics Fundamentals • Creating and Configuring a Google Analytics Account • Monitoring Campaigns with Google Analytics Reports • Setting Goals with Google Analytics • Analysing and Recording Google Analytics Data • Using Google Analytics 4 Content Marketing: This module teaches you how to create content that speaks to people, at the right time and via the right channels. Understand what works for you by tracking and measuring performance. • Content Marketing Concepts and Strategy • Developing a Content Marketing Plan • Publishing and Distributing Content • Using Content Research to Find Opportunities • Creating and Curating Content • Metrics and Performance • Establishing Content Intent Search Engine Optimisation (SEO): Search Engine Optimisation is all about getting on that first Google page, staying top of the list and top of mind. Develop keyword strategies, understand the kind of content that attracts users, optimise rankings and then convert visitors to customers. • SEO Fundamentals • Keywords and SEO Content Plan • Measuring SEO Performance • Aligning SEO and Business Objectives • Optimise Organic Search Ranking Display and Video Advertising: Learn how to set up, manage and optimise your YouTube channel. Target, test and develop your use of the Google Display Network and get creative with visual formats. • Fundamentals of Display and Video Advertising • Google Display Network and Video Ad Formats • Creating and Managing a YouTube Channel • Creating Display and Video Campaigns • Targeting Display and Video Campaigns • Measurement and Optimisation • Reporting on Display Campaign Website Optimisation: What makes a winning website? This module gives you the skills to create a simple, well-designed, optimised WordPress site that not only looks good but also delivers for your business. • Web Design and Website Optimisation • Design Principles and Website Copy • Publishing a Basic Website • User-Centered Design and Website Optimisation • Website Metrics and Developing Insight Digital Marketing Strategy: Underneath your digital activity, you need solid objectives. This module helps you to build a clear vision of your strategy, and makes it actionable with budget, channel and media plans, KPIs and more. • Digital Strategy Fundamentals • Setting Strategy Objectives and KPIs • Digital Strategy Research • Developing a Creative Strategy • Executing a Digital Marketing Strategy • Communicating a Digital Marketing Strategy • Forecasting performance DURATION 8-10 Weeks WHATS INCLUDED Course Material Case Study Experienced Lecturer Refreshments Certificate

FORS Virtual Reality Safe Driving - Periodic 7 Hour CPC Course - Wakefield - Dec 2025
By Total Compliance
Experience safer urban roads with our FORS Approved Virtual Reality Safe Urban Driving Training. Our immersive program empowers drivers to navigate bustling city streets alongside vulnerable road users, fostering a culture of road safety. With a seven-hour DCPC-approved course, participants gain valuable insights and skills for responsible driving. Invest in your drivers' safety and meet compliance requirements while protecting lives on the road. Elevate road safety with our innovative VR training. Learn more at www.totalcompliance.co.uk

QA Level 2 Award In Working At Height (RQF) Face to Face: Half-day course Virtual Classroom: Spread over 2 sessions of 2½ hr duration Raises awareness of safety considerations whilst working at height Teaches how to ensure a safe working environment Course Contents: Legislation, regulations, roles and responsibilities when working at height The consequences of non-compliance Safe working practices when working at height How to reduce the risks from:Weather conditionsWorking environmentsOthers working at heightFalling objectsFragile surfaces Importance of risk assessments Rescue plan requirements Selecting appropriate equipment Identifying safety precautions when using equipment Importance of Personal hygiene Reporting procedures when equipment is faulty Benefits of this course: 35 People died in 2017/18 due to falls from height There were also 43,000 non-fatal accidents due to falls from height These resulted in 80 major injuries And 230 over-3-day absences, costing employees and employers money Even just a height of less than 2 meters can result in serious, or even fatal, injuries The Work at Height Regulations 2005 require employers, and those in charge of any type of work at height activity to ensure that the work is properly planned, well supervised and carried out by competent people Employees who do this course will learn to have a greater awareness of the risks involved in working at height, and on how to best manage those risks, ensuring the safety of all concerned Accredited, Ofqual regulated qualification: This Health and Safety Training Course is a nationally recognised, Ofqual regulated qualification accredited by Qualsafe Awards.This means that you can be rest assured that your Health and Safety Certificate fulfils the legal requirements and is an excellent way to make sure you and your employees are trained in Health and Safety.The Ofqual Register number for this course is 603/2687/6

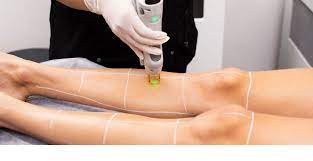
Laser Course Package 1
By FNBC Training Academy
Laser core of knowledge
CPCS A09 A Forward Tipping Dumper- Wheeled
By M.I Construction Training
CPCS A09

FORS Safe Driving - Periodic 7 Hour CPC Course -Birmingham - Jan 2026
By Total Compliance
#SafeUrbanDriving #Birmingham #driver #driver_safety #driver_training #fors #nottingham #sud

Search By Location
- regulation Courses in London
- regulation Courses in Birmingham
- regulation Courses in Glasgow
- regulation Courses in Liverpool
- regulation Courses in Bristol
- regulation Courses in Manchester
- regulation Courses in Sheffield
- regulation Courses in Leeds
- regulation Courses in Edinburgh
- regulation Courses in Leicester
- regulation Courses in Coventry
- regulation Courses in Bradford
- regulation Courses in Cardiff
- regulation Courses in Belfast
- regulation Courses in Nottingham