- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
5088 Courses
Quality Assurance for Good Laboratory Practice
By Research Quality Association
Course Information A must-have programme for Quality Assurance auditors stepping into or honing their role within a Good Laboratory Practice (GLP) environment, this course offers invaluable, expert guidance for crafting a robust and efficient GLP audit programme. What will I learn? A solid regulatory foundation underpinning quality assurance activities Clarity on the roles of Quality Assurance, management, and study director within the framework of Good Laboratory Practice principles Enhanced efficacy in inspections and audits Heightened compliance with Good Laboratory Practice standards for your facility Unique insights into governmental monitoring activities within the GLP sphere. This course is structured to encourage delegates to Discuss and develop ideas Solve specific problems Examine particular aspects of GLP. Tutors Tutors will be comprised of (click the photos for biographies): Cate Ovington Director, The Knowlogy Group Ltd Jane Elliston Senior Quality Assurance Auditor, Battelle UK Shona Ross Head of QA, Tower Mains Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Welcome and Introductions 09:15 Good Laboratory Practice Standards and Regulations An insight into the background and history of Good Laboratory Practice. 09:45 Principles of Quality Assurance What is the role and responsibilities of QA in GLP. Maintaining the independence of QA and what is an audit. 10:30 Break 10:45 Standard Operating Procedures GLP requirements and QA involvement. 11:30 Study Plans GLP requirements and QA involvement. 12:05 QA Programme Risk based programme, what are study, process and facility audits. 13:00 Lunch 14:00 Inspections Attitudes, techniques and attributes. 14:40 Workshop 1 - Facility and Process Inspections An exercise in inspection planning and preparation for inspections. 15:15 Break 15:30 Workshop 1 - Feedback 15:45 The Auditor and Audit Conduct Attitudes, attributes and techniques. 16:30 Panel Session An opportunity for delegates to put questions to the panel of speakers. 17:15 Close of Day Day 2 09:00 Workshop 2 - A Mock Audit 10:45 Break 11:00 Workshop 2 - Feedback 11:30 Auditing the Study Report Techniques and methods for the QA audit of the study report. 12:00 Record Keeping and Data The impact of GLP on data and records management. 12:40 Lunch 13:25 Data Integrity A look at the OECD GLP guidance document; the expectations of the regulators and the involvement of QA - Where QA adds value. 14:15 Workshop 3 - Amendments to Study Plan and Deviations from the Plan What are they? What is the difference between them? How are they controlled? 15:00 Workshop 3 - Feedback 15:15 Break 15:30 Regulatory Compliance GLP Monitoring Authority monitoring for compliance with Good Laboratory Practice. 16:15 Panel Session An opportunity for delegates to put questions to the panel of speakers. 16:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Learn

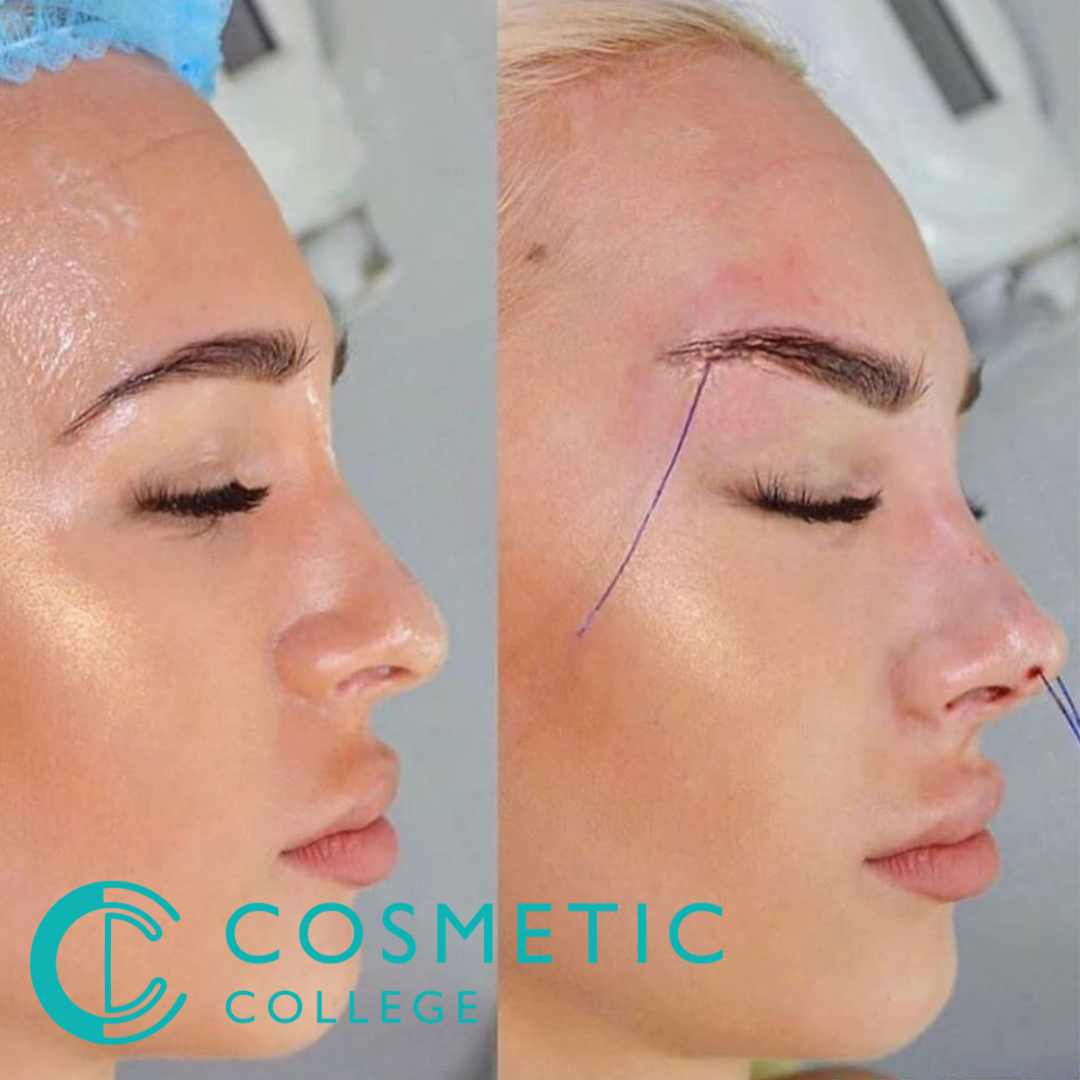
Fox Eye & Pixie Lift Thread Training Course
By Cosmetic College
This treatment is an innovative technique which uses dissolvable sutures to lift and elongate the eye to create a more almond shape whilst lifting the brow tail to create a feminine effect. This non-invasive procedure works by inserting a blunt cannula and a dissolvable thread discreetly under the surface of the skin. The thread provides a source of tension which will gradually attach itself to the tissue and stimulate the body's collagen production. Additionally included in this course you will learn how to provide pixie nose lift treatments for your clients. This technique is popular as it's the most cost-effective and offers a great option for those wanting to build up the effect of the lift over time. Course Entry Requirements: This course is suitable for those with or without a medical background. It is designed to provide the student with the ability to seek employment or start their own business upon qualification. At a minimum, students will be required to be qualified for at least one of the following: Medically qualified as a nurse, doctor or dentist with current registration with the NMC, GMC or GDC. NVQ Level 3 in Beauty Therapy, ITEC or HND 12 months of needling experience 6 Months of micropigmentation experience and Anatomy & Physiology Level 3 If your qualification does not appear above, we offer a fast track access course for those completely new to the industry. Course agenda Background of PDO Threads Health & safety In-depth anatomy and physiology Emergency protocols Product knowledge Sourcing clinical oversight (Prescriber) Complications prevention Client suitability Equipment use Needle stick injury protocol Pain management with the use of injectable anaesthetic Adverse effects Complications management Emergency Protocols Anaphylaxis Aftercare Consent forms Consultation process Client selection Live demonstrations Live model experience Recommended treatment charges Insurance Legalities Advance your training with our complete PDO Threads training package Take your training to the next level by enrolling on our complete PDO Thread training package. Included within this package: PDO COG Threads PDO Mono Threads Fox Eye Thread Lifts Pixie Nose Tip Lifts Check out the package here Course Benefits Student Benefits Advanced Skill Development: This course provides students with specialised training in the techniques of thread lifting specifically for creating the fox eye and pixie lift effects. It allows students to expand their skill set and offer unique and in-demand treatments to their clients. Increased Client Satisfaction: With the growing popularity of the fox eye and pixie lift trends, clients are actively seeking practitioners who can deliver these desired outcomes. By mastering these techniques, students can meet their client's expectations and provide them with the desired eye and facial rejuvenation results. Higher Earning Potential: As students acquire specialised skills in the fox eye and pixie lift thread techniques, they can command higher fees for these advanced treatments. The ability to offer unique and sought-after procedures often translates into increased earning potential for practitioners. Career Advancement Opportunities: With expertise in the fox eye and pixie lift thread techniques, students may have more opportunities for career advancement. Client Benefits Expertise in Trending Procedures: Clients can be confident that their practitioner is trained and knowledgeable in the specific techniques required to achieve the fox eye and pixie lift effects. This expertise increases the likelihood of achieving desired results. Personalised Treatment Plans: Practitioners who have completed this training can provide clients with tailored treatment plans based on their unique facial features and desired outcomes. This customised approach helps ensure client satisfaction. Safe and Effective Procedures: Clients can trust that their practitioner is skilled in performing thread lifting procedures safely and effectively, minimising the risk of complications and ensuring a positive treatment experience. Natural-looking Results: The fox eye and pixie lift thread techniques aim to enhance natural beauty and create subtle yet noticeable improvements. Clients can expect results that look natural, youthful, and in harmony with their overall facial aesthetics. Earning Potential The earning potential and salary expectation for practitioners who have completed the Fox Eye & Pixie Lift Thread Training Course can vary based on several factors. These factors include the practitioner's location, years of experience, client base, and the pricing structure of their services. By acquiring the skills and expertise in performing the fox eye and pixie lift thread techniques, practitioners can attract clients who are specifically interested in these procedures. This specialisation and ability to meet the growing demand for these treatments can contribute to higher earning potential.
Neurodiversity Workshop Navigating the Workplace
By Mpi Learning - Professional Learning And Development Provider
A four-hour workshop for Neurodiverse individuals and those who recognise some traits in themselves.

Power BI® – Business Data Analytics
By EnergyEdge - Training for a Sustainable Energy Future
Discover Power BI business data analytics through EnergyEdge's course. Enhance your skills and gain valuable insights for your business.
QA Level 3 Award In Emergency Paediatric First Aid (RQF) Full day course Would you know what to do if you saw a child in need of First Aid? Being able to deal with paediatric emergencies can make the difference between the life and death of children, and save them a lot of suffering Course Contents: The Roles and Responsibilities of an Emergency Paediatric First Aider Assessing an Emergency Situation Accident Recording Minor Injuries Cuts, Grazes and Bruises Minor Burns and Scalds Managing an Unresponsive Infant or Child Recovery Position CPR Safe Use of an AED (Automated External Defibrillator) Choking Anaphylaxis Seizures Wounds and Bleeding Shock Benefits of this course: Would you know what to do if you saw a child in need of First Aid? Children are prone to minor injuries, but suffer from serious injuries also In 2014, 2,269 children in the UK were so badly bitten by an animal they had to be admitted to hospital More than 2 million children have accidents in the home for which they're taken to A&E - every year, with Under 5s accounting for 7% of all hospital emergency treatments Being able to deal with peadiatric emergencies can make the difference between the life and death of children, and save them a lot of suffering. This QA Level 3 Award in Emergency Paediatric First Aid (RQF) qualification is ideal for: - Parents/carers or family members who want to learn key paediatric first aid skills - Those who work with children and are not required to comply with Ofsted’s Childcare Register or Early Years Foundation Stage (EYFS) 2014 requirements Those who want to provide additional support in their organisation to existing paediatric first aiders that are trained to meet Ofsted’s Childcare Register or Early Years Foundation Stage (EYFS) 2014 requirements. For those who will be directly responsible for children, Ofsted requires people to do our two day Paediatric First Aid course instead We also run a Paediatric Annual Refresher to keep those life-saving skills up to date Accredited, Ofqual regulated qualification: Our Emergency Paediatric First Aid at Work course is a nationally recognised, Ofqual regulated qualification accredited by Qualsafe Awards. This means that you can be rest assured that your Emergency Paediatric First Aid certificate will fulfill the legal requirements. It is a very good way to make sure you and your employees are trained in First Aid for Children and Infants (babies). The Ofqual Register number for this course is 603/0786/9

Certified Scrum Professional-ScrumMaster: In-House Training
By IIL Europe Ltd
Certified Scrum Professional®-ScrumMaster® (CSP®-SM): In-House Training Certified Scrum Professionals challenge their teams to improve the way Scrum and Agile principles are applied. They have demonstrated experience, documented training, and proven knowledge in Scrum. Are you ready to take your knowledge and skillset in your role as Scrum Master to the next level? If so, it's time to elevate your career further by earning the Certified Scrum Professional®-ScrumMaster (CSP®-SM) certification. What you will Learn Learn to find practical solutions and improve your implementation of Scrum in the workplace. Aside from the pride gained and earning potential of attaining CSP® level, you can also: Attend exclusive CSP® events with other leaders in Scrum and Agile Attract more recruiters and command a higher rate of pay Establish a gateway and milestone toward becoming CST®, CEC, or CTC Receive a free premium subscription to the world's largest Agile assessment and continuous improvement platform, Comparative Agility®

A System Approach to Good Pharmacovigilance Practice
By Research Quality Association
Course Information In today's evolving landscape, pharmacovigilance (PV) systems face ongoing challenges due to global, national, and company-specific events. This course focuses on developing personnel equipped to navigate these complexities and improve the safe use of medicinal products. We emphasise continuous global thinking, communication, and strategic planning, ensuring adaptability across various levels. The course explores maintaining PV system functionality while adhering to regulatory requirements. Participants will apply European regulatory standards to enhance and sustain PV system effectiveness, contributing to improvement initiatives and ensuring operational integrity. The course covers PV system intricacies, regulatory compliance, and a 'systems approach' for auditors, quality assurance personnel, and PV practitioners. Through presentations and workshops, attendees gain insights into implementing and maintaining an effective PV system. Who should attend? Auditors Pharmacovigilance Quality System Managers Pharmacovigilance scientists The QPPV. Course benefits Throughout the course delegates will explore application of the legal requirements for the PV system and quality system and how to assure these systems. They will demonstrate their ability to contribute to: A systematic investigation of the pharmacovigilance system and its quality system Examination of how the pharmacovigilance system and quality system interact to achieve compliance. The risk-based approach to auditing the PV system and quality system The maintenance of 'inspection readiness' Explore how to investigate the complex PV system Discussions about how to monitor and maintain the PV system and assure compliance. Course Objectives Clarify what has to be done: Explore application of the legal requirements. Explore how to do what has to be done: Adopt a systemic approach to systematically investigate or implement and maintain the PV system and quality system Examine how a compliant PV system and a compliant quality system interact to achieve compliance with regulatory requirements for PV Explore how to investigate the complexity of the PV system. Discus how to identify what is missing or what needs to be improved: Discuss how to monitor and maintain the PV system and assure compliance. This course will assist delegates with: An understanding of key system principles, A practical approach to implementing, maintaining and monitoring the PV system and its quality system A procedure to share expertise to increase efficiency and confidence. This course is structured to encourage delegates to: Discuss and develop ideas - Share knowledge and experiences - Solve specific problems. By the end of the course delegates will be able to: Understand better the pharmacovigilance system, its quality system and how the components interact to achieve the objectives of pharmacovigilance Investigate, and analyse the pharmacovigilance system and to identify what is missing and what needs to be improved. Tutors Tutors will be comprised of (click the photos for biographies): Jana Hyankova Head of PV Department, IVIGEE Services a.s. Programme Please note timings may be subject to alteration. Day 1 08:30 Welcome, registration, course objectives and introduction to work groups Housekeeping notices, meet other delegates, explore how to work in your work group, course objectives. Clarify the definition and objectives of Pharmacovigilance. 09:30 The Regulatory Framework for Pharmacovigilance Identify the relevant regulations and directives. Explore GVP guidance, structure of the modules and standard format of each module. 10:00 The Pharmacovigilance System Exploration of how to organise what has to be done, communications. Exploration of the structures and processes for pharmacovigilance. 10:30 Break 11:00 Workshop 1 and Feedback Exploring an organisational model of the pharmaceutical company- cooperation between PV and other stakeholders. 12:00 The Quality System for pharmacovigilance Exploration of the structures, processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 13:00 Lunch 14:00 Workshop 2 and Feedback The quality system puzzle. Explore the organisation of the PV quality system and how it interacts with the PV system. 14:30 The Quality System for pharmacovigilance Exploration of the structures processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 15:00 Description of PV System 15:30 Break 15:30 Workshop 3 and Feedback The quality system puzzle Explore the organisation of the PV quality system and how it interacts with the PV system. 16:00 The Pharmacovigilance Safety Master File Construction of the Pharmacovigilance System Master File and its purpose. 17:00 Workshop 3 and Feedback Description of PV System. 18:00 End of Day Day 2 08:30 Drug Safety in the Clinical Trial Environment - Part 1 Information flow and responsibilities of the sponsor. 09:30 Workshop 4 and Feedback Drug Safety in the Clinical Trial environment: Information flow and responsibilities of the sponsor. 10:30 Break 11:00 Drug Safety in the Clinical Trial Environment - Part 2 Information flow and responsibilities of the sponsor. 12:00 Lunch 13:00 Workshop 5 and Feedback Drug Safety in the clinical trial environment: Information flow and responsibilities of the sponsor. 13:30 Processing of Safety Data Exploration of safety data processing, verification, validation, follow up, formatting and collation, reporting requirements, quality and data management. 15:00 Break 15:30 EudraVigilance Exploration of how EudraVigilance supports the PV system. 16:15 Signal Detection and Evaluation/Risk Benefit Assessment: Pharmacovigilance Risk Assessment Committee (PRAC): What is a signal? What are the regulatory requirements? How is signal detection and evaluation conducted? Qualitative and quantitative methods of signal detection. Risk benefit assessment. 17:00 Risk Management Plans A cornerstone of Pharmacovigilance safety communications, direct healthcare professional communication 18:00 End of Day Day 3 08:30 The Pharmacovigilance Risk Assessment Committee (PRAC) Exploration of how good practice is achieved. Composition, role and responsibilities. Examples of referrals. 09:15 Development Safety Update Reports (DSURs): Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:30 Break 11:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 12:00 Workshop 6 and Feedback To explore the compilation and submission of the PSUR. 13:00 Lunch 13:30 Role of the QPPV Exploration of the legal responsibilities of the QPPV and the MAH. 14:30 Break 15:00 Workshop 7 and Feedback To explore the challenges faced by the QPPV. 15:30 End of course Extra Information Face-to-Face Course Course material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device< Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD Points 23 Points Development Level Develop

An Understanding of Pressure Area Care
By Guardian Angels Training
Enhance your knowledge and skills in pressure area care with our comprehensive course. Ideal for healthcare professionals, caregivers, and nurses.

Building High-Performance Teams: In-House Training
By IIL Europe Ltd
Building High-Performance Teams: In-House Training This course pulls together the most current and popular theories and writings on this complex topic and presents this amalgamated view in a highly interactive workshop and activity-based approach. Students will understand and have the skills required to build and participate in high-performance project teams and will possess the insight to proactively affect change within their respective organizations by guiding the existing culture to one that promotes high performance. What you will Learn At the end of this program, you will be able to: Define a team and describe the optimum team size for effective performance Describe characteristics and guiding behaviors of high-performance teams Describe the major elements of each development stage in two distinct models Recognize cultural barriers in achieving high performance List the attributes of a high-performing corporate culture Assess your own corporate culture Discuss corporate leadership as a factor in building high-performance project teams Describe the three A's of selecting team members State three leadership responsibilities Describe leadership responsibilities, styles, and roles List and describe the eight components of the team charter model for building high-performance teams Foundation Concepts The Nature of Teams Characteristics of High-Performance Teams Understanding Team Development Stages of Team Development - Model 1 Stages of Team Development - Model 2 Designing a High-Performance Culture Corporate Cultures Corporate Leadership Establishing the Attributes of High Performance Choosing the Right People Team Effectiveness Team Leadership The Team Charter Model

Search By Location
- Management Courses in London
- Management Courses in Birmingham
- Management Courses in Glasgow
- Management Courses in Liverpool
- Management Courses in Bristol
- Management Courses in Manchester
- Management Courses in Sheffield
- Management Courses in Leeds
- Management Courses in Edinburgh
- Management Courses in Leicester
- Management Courses in Coventry
- Management Courses in Bradford
- Management Courses in Cardiff
- Management Courses in Belfast
- Management Courses in Nottingham
