- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
517 Courses
Emergency First Aid at Work (1-Day)
By Prima Cura Training
The Health and Safety (First Aid) Regulations 1981 Emergency First Aid at Work
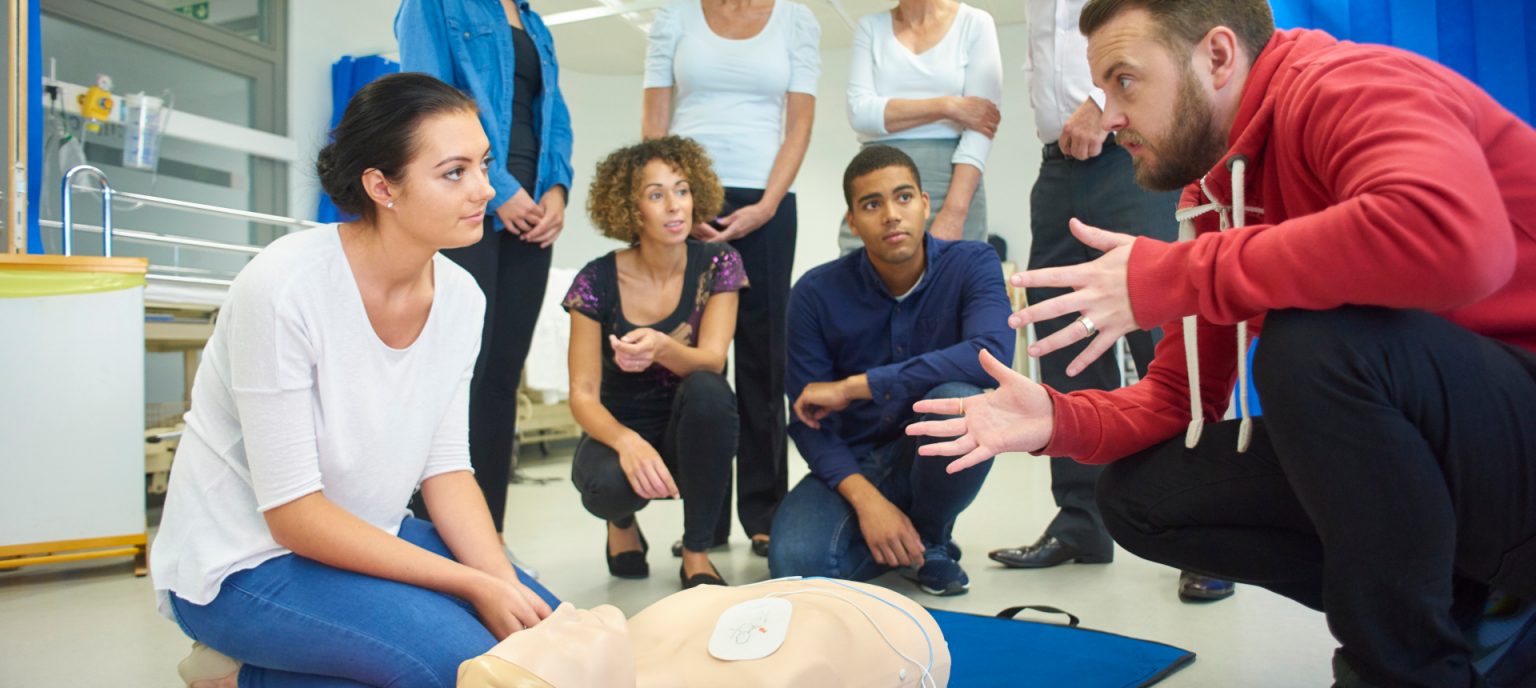
Level 4 Laser and IPl Treatments
By Aesthetic Laser Training Academy
Laser Hair Removal and Skin Rejuvenation
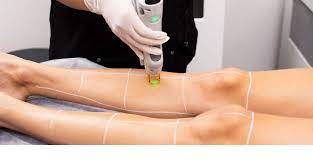
Good Laboratory Practice for Study Directors, Principal Investigators, Study Staff and Management
By Research Quality Association
Course Information Embark on our GLP course offering extensive guidance and pragmatic support tailored for individuals serving as Study Directors or Principal Investigators overseeing non-clinical safety studies on pharmaceuticals, agricultural, and industrial chemicals within the realm of Good Laboratory Practice (GLP). This comprehensive programme extends its benefits to study staff and management operating in GLP-compliant environments. The course extensively covers the current OECD GLP Principles and UK GLP legislation, while also referencing international standards, regulations, and guidelines pertinent to the field. Benefits of this course: Practical help and guidance on the interpretation and application of GLP An opportunity to update your knowledge of GLP with the current interpretation of requirements Access to an experienced panel of speakers Information on how other organisations address GLP issues An opportunity to improve your understanding of the GLP requirements as they are applied in different situations. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of GLP Learn from the experience of others. Tutors Tutors will be comprised of (click the photos for biographies): Tim Stiles Consultant, Qualogy Ltd Tony Woodall Head of Quality Assurance, Alderley Analytical Gill Armour Study Monitor Team Leader, AstraZeneca Jane Elliston Senior Quality Assurance Auditor, Battelle UK Vanessa Grant -, - Jeanet Logsted CEO, Scantox Programme Please note timings may be subject to alteration. Day 1 09:00 Registration 09:15 Welcome and Introductions 09:35 Development of Good Laboratory Practice A review of the history of GLP, its current scope and application, with a synopsis of current European and international standards. 10:05 Roles and Responsibilities The responsibilities of study director, test facility, management and study staff in the conduct of a GLP study. 10:45 Break 11:00 The Roles and Responsibilities of the Study Director and Test Facility Management The role of the study director in the management and control of a study, as defined by GLP, and management's roles are explored. 11:45 Multi-site Studies What is a multi-site study and when should such concepts be applied on a study. The role of the study director and principal investigator in the planning, conduct and reporting of multi-site study are explored. 12:30 Study Plan (Protocols) GLP requirements for the preparation of a study plan, content, authorisation, amendments and deviations are discussed. 13:00 Lunch 13:45 Workshop 1 - The Study Plan Some practical problems with study plans and amendments explored. 14:45 Workshop 1 - Feedback 15:00 Standard Operating Procedures The control, content and authorisation of SOPs and the principles behind the practice. 15:30 Break 15:45 Workshop 2 - Practical Study Conduct Problems Dealing with practical problems encountered during the conduct of studies. 16:40 Workshop 2 - Feedback 17:15 Close of Day Day 2 09:00 Questions and Answers Discussion of issues raised by course delegates. 09:20 Quality Assurance The interactions between QA, management, study director and principal Investigator are discussed as is QAs role when conducting a multi-site study. 10:00 The Final Report The content of the final report and the role of those involved in its preparation and approval. Specific reporting requirements when conducting a multi-site study are also explained. 10:30 Break 10:45 Workshop 3 - Final Report Problems Practical problems of report preparation including compliance statements. 11:30 Workshop 3 - Feedback 12:00 Management of Raw Data and Records A view on how records and materials are managed and archived in compliance with GLP. 12:45 Lunch 13:30 Workshop 4 - Data and Sample Management Issues Dealing with data and sample management issues. 14:15 Workshop 4 - Feedback 14:45 Regulatory Inspection Government monitoring for compliance with Good Laboratory Practice. 15:15 Panel Session This panel session will address any outstanding issues raised by delegates. 15:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

SIA Door Supervisor Course + First Aid
By London Construction College
Take The SIA Door Supervisor Course + First Aid, This Will Take You 6 Days To Complete. Enrol Now On To The Course! What Is SIA Door Supervisor Course? The SIA (Security Industry Authority) Door Supervisor Training is a comprehensive training program designed to prepare individuals for roles as door supervisors within the security industry in the United Kingdom. Door supervisors play a critical role in ensuring safety and security at licensed premises, including bars, clubs, and events. This SIA Door Supervisor Course + First Aid is 6 days, this is a compulsory course in order to attain the Door Supervisor Licence. Furthermore, this will allow you to work as a doorman, nightclub security, retail security, corporate security, construction security and many other security sectors. Enrol now or contact us for any further details. Course Overview: 6 Days Course | 09:00 – 18:30 | Mon – Sat Every Week SIA Door Supervisor Course Road Map 1. Book Course Book your SIA Door Supervisor Training and First Aid Course in London (6 Days) 2. Attend Course Attend a 6-day course, and successfully pass all your SIA Door Supervisor exams. 3. Apply SIA Register for an account with SIA, and apply for your SIA Door Supervisor Licence. Course Information Why Choose SIA Door Supervisor Course? Expert Instructors Learn from seasoned professionals who bring real-world insights to the classroom. Our instructors are dedicated to your success, offering guidance and support throughout the training. Practical Training Gain hands-on experience in simulated security scenarios, preparing you for the challenges you’ll face in the field. Our practical sessions enhance your problem-solving skills and decision-making abilities SIA Compliance Stay ahead in the security industry by understanding and complying with SIA regulations. Our course covers the latest guidelines, ensuring you are well-versed in the legal and ethical aspects of security operations Job Placement Assistance We go beyond training by offering job placement assistance. Our network of industry connections helps you kickstart your security career with confidence. Ready To Begi Your SIA Journey? Your journey toward a rewarding and responsible career as a Door Supervisor starts here. Contact us today to enrol in our Door Supervisor Training at London Construction College, and take the first step towards an exciting and impactful career in security. Your future awaits! SIA Door Supervisor Course Content Unit 1 : Working In The Private Security Industry Legal Considerations in the Private Security Industry. Ensuring Health and Safety for Private Security Operatives . Awareness of Fire Safety. Procedures for Emergency Situations. Effective Communication Skills and Customer Care. Unit 2: Working As A Door Supervisor Adherence to Behavioral Standards. Understanding Civil and Criminal Law. Procedures for Search Operations. Protocols for Arrest Situations. Awareness of Drugs and Their Impact. Documentation of Incidents and Preservation of Crime Scenes. Compliance with Licensing Laws. Emergency Procedures. Unit 3: Conflict Management For The Private Security Industry Conflict Avoidance and Personal Risk Mitigation. De-escalation of Conflicts. Resolution and Lessons from Conflicts . Application of Communication and Conflict Management Skills for Door Supervisors. Unit 4: Physical Intervention Skills For The Private Security Industry Overview of Physical Intervention Skills. Essential Legislation Awareness . Fun Fact: The persuasive Communication and Conflict Management skills acquired in this course are applicable both professionally and personally in conflict situations. This standalone component alone holds a value exceeding the course price. SIA Door Supervisor Exam On the last day of the course, you’ll encounter four multiple-choice exams along with a practical assessment. We acknowledge that exams can be intimidating, but rest assured, there’s no cause for concern. The Door Supervisor course comprehensively covers all exam topics, and your skilled instructor will thoroughly equip you for success in these assessments. Embarking on an exciting career in the security industry as a Door Supervisor or Security Guard starts with the initial step of acquiring the right training. Throughout your Door Supervisor training, we guide you through the entire process, offering essential training and providing the necessary materials to assist you in obtaining your SIA Door Supervisor license. Document Checks Proof Of Identity You will need to provide documents that prove you are who you say you are. Address history You need to provide two proofs of address. Bank or building society statement issued within the last three months. Utility bill issued within the last three months. A credit card statement was sent to your current address within the last three months. Council Tax statement issued in the last 12 months. Mortgage statement issued in the last 12 months. Age You must be 18 or over to hold an SIA licence. Criminal record Please be aware, that even if you pass the SIA door supervisor, you will still need to pass SIA criminal record checks. If you live outside the UK, or you have spent 6 continuous months or more outside the UK in the last 5 years, you need to provide evidence of overseas criminal record checks to the SIA. SIA will only issue a licence after completing background checks. Right to work in the UK You must have the right to work in the UK to get a front-line or non-front-line licence. Important Things You Should Know FAQ SIA Door Supervisor Training What is the SIA Door Supervisor course, and why do I need it? The SIA Door Supervisor course is a training program designed for individuals seeking employment in the security industry, specifically in roles such as door supervision. The Security Industry Authority (SIA) requires this qualification for those working in designated roles to ensure a standard level of competence and professionalism. What does the SIA Door Supervisor Course cover? The SIA course covers a range of topics essential for door supervisors, including conflict management, physical intervention, emergency procedures, legal responsibilities, and customer service. It provides a comprehensive skill set needed to excel in the role. Can I take the course in London? Yes, the SIA Door Supervisor course is available in London. How long does the course take to complete? The SIA Door Supervisor and First Aid is completed over 6 days of training. Is there an age requirement for taking the SIA Door Supervisor course? Yes, you must be at least 18 years old to take the SIA Door Supervisor course as it is a legal requirement for working in the security industry. What are the job prospects after completing the course? Successfully completing the SIA Door Supervisor course enhances your employability in the security industry. Door supervisors are in demand in various establishments, including nightclubs, bars, and events, and having the SIA qualification opens up job opportunities in these sectors. Do I need any prior experience in security to enrol in the course? No, prior experience in security is not a prerequisite for enrolling in the SIA Door Supervisor course. The course is designed to provide comprehensive training for individuals at various levels, including those new to the security industry. Can I take the SIA Door Supervisor course if I’m not a UK citizen? Yes, the SIA Door Supervisor course is open to individuals regardless of nationality. However, it’s essential to ensure that you meet the legal requirements for working in the UK, including visa regulations.

ALLMI Lorry Loader -2 Day Novice Course | Milton Keynes | 16 - 17 May 2025
By Total Compliance
Equipment used - Lorry mounted cranes and Hook attachments. Who Would Do This Training? Anyone who operates or intends to be operating lorry mounted cranes (sometimes referred to as HIAB’s) should undertake training as is required by the Lifting Operations and Lifting Equipment Regulations 1998. This course is made to suit novice operators, and are designed to ensure the efficient and safe operation of equipment. HIAB is really a brand name and the correct term for the equipment is ‘lorry mounted crane’ or ‘lorry loader crane’ (when mounted to a commercial vehicle). Other manufacturers of these types of cranes include Atlas, Cormach, Fassi, HMF, Hyva, Palfinger, Penny Hydraulics and PM. Which Industries Train with ALLMI? The ALLMI Lorry Loader Course is recognised by CSCS. The Lorry Loader Crane courses and Slinger Signaller courses, certificated by ALLMI, cover a wide variety of hydraulic cranes, both manually and electronically operated. Manuals and Postage of ALLMI Certificate and Card are included in your Ticket Price

ALLMI Lorry Loader -2 Day Novice Course | Milton Keynes | 6 - 7 June 2025
By Total Compliance
Equipment used - Lorry mounted cranes and Hook attachments. Who Would Do This Training? Anyone who operates or intends to be operating lorry mounted cranes (sometimes referred to as HIAB’s) should undertake training as is required by the Lifting Operations and Lifting Equipment Regulations 1998. This course is made to suit novice operators, and are designed to ensure the efficient and safe operation of equipment. HIAB is really a brand name and the correct term for the equipment is ‘lorry mounted crane’ or ‘lorry loader crane’ (when mounted to a commercial vehicle). Other manufacturers of these types of cranes include Atlas, Cormach, Fassi, HMF, Hyva, Palfinger, Penny Hydraulics and PM. Which Industries Train with ALLMI? The ALLMI Lorry Loader Course is recognised by CSCS. The Lorry Loader Crane courses and Slinger Signaller courses, certificated by ALLMI, cover a wide variety of hydraulic cranes, both manually and electronically operated. Manuals and Postage of ALLMI Certificate and Card are included in your Ticket Price

ALLMI Lorry Loader -2 Day Novice Course | Milton Keynes | 1 - 2 August 2025
By Total Compliance
Equipment used - Lorry mounted cranes and Hook attachments. Who Would Do This Training? Anyone who operates or intends to be operating lorry mounted cranes (sometimes referred to as HIAB’s) should undertake training as is required by the Lifting Operations and Lifting Equipment Regulations 1998. This course is made to suit novice operators, and are designed to ensure the efficient and safe operation of equipment. HIAB is really a brand name and the correct term for the equipment is ‘lorry mounted crane’ or ‘lorry loader crane’ (when mounted to a commercial vehicle). Other manufacturers of these types of cranes include Atlas, Cormach, Fassi, HMF, Hyva, Palfinger, Penny Hydraulics and PM. Which Industries Train with ALLMI? The ALLMI Lorry Loader Course is recognised by CSCS. The Lorry Loader Crane courses and Slinger Signaller courses, certificated by ALLMI, cover a wide variety of hydraulic cranes, both manually and electronically operated. Manuals and Postage of ALLMI Certificate and Card are included in your Ticket Price

ALLMI Lorry Loader -2 Day Novice Course | Milton Keynes | 11 - 12 July 2025
By Total Compliance
Equipment used - Lorry mounted cranes and Hook attachments. Who Would Do This Training? Anyone who operates or intends to be operating lorry mounted cranes (sometimes referred to as HIAB’s) should undertake training as is required by the Lifting Operations and Lifting Equipment Regulations 1998. This course is made to suit novice operators, and are designed to ensure the efficient and safe operation of equipment. HIAB is really a brand name and the correct term for the equipment is ‘lorry mounted crane’ or ‘lorry loader crane’ (when mounted to a commercial vehicle). Other manufacturers of these types of cranes include Atlas, Cormach, Fassi, HMF, Hyva, Palfinger, Penny Hydraulics and PM. Which Industries Train with ALLMI? The ALLMI Lorry Loader Course is recognised by CSCS. The Lorry Loader Crane courses and Slinger Signaller courses, certificated by ALLMI, cover a wide variety of hydraulic cranes, both manually and electronically operated. Manuals and Postage of ALLMI Certificate and Card are included in your Ticket Price

ALLMI Lorry Loader -2 Day Novice Course | Milton Keynes | 27 - 28 June 2025
By Total Compliance
Equipment used - Lorry mounted cranes and Hook attachments. Who Would Do This Training? Anyone who operates or intends to be operating lorry mounted cranes (sometimes referred to as HIAB’s) should undertake training as is required by the Lifting Operations and Lifting Equipment Regulations 1998. This course is made to suit novice operators, and are designed to ensure the efficient and safe operation of equipment. HIAB is really a brand name and the correct term for the equipment is ‘lorry mounted crane’ or ‘lorry loader crane’ (when mounted to a commercial vehicle). Other manufacturers of these types of cranes include Atlas, Cormach, Fassi, HMF, Hyva, Palfinger, Penny Hydraulics and PM. Which Industries Train with ALLMI? The ALLMI Lorry Loader Course is recognised by CSCS. The Lorry Loader Crane courses and Slinger Signaller courses, certificated by ALLMI, cover a wide variety of hydraulic cranes, both manually and electronically operated. Manuals and Postage of ALLMI Certificate and Card are included in your Ticket Price

A System Approach to Good Pharmacovigilance Practice
By Research Quality Association
Course Information In today's evolving landscape, pharmacovigilance (PV) systems face ongoing challenges due to global, national, and company-specific events. This course focuses on developing personnel equipped to navigate these complexities and improve the safe use of medicinal products. We emphasise continuous global thinking, communication, and strategic planning, ensuring adaptability across various levels. The course explores maintaining PV system functionality while adhering to regulatory requirements. Participants will apply European regulatory standards to enhance and sustain PV system effectiveness, contributing to improvement initiatives and ensuring operational integrity. The course covers PV system intricacies, regulatory compliance, and a 'systems approach' for auditors, quality assurance personnel, and PV practitioners. Through presentations and workshops, attendees gain insights into implementing and maintaining an effective PV system. Who should attend? Auditors Pharmacovigilance Quality System Managers Pharmacovigilance scientists The QPPV. Course benefits Throughout the course delegates will explore application of the legal requirements for the PV system and quality system and how to assure these systems. They will demonstrate their ability to contribute to: A systematic investigation of the pharmacovigilance system and its quality system Examination of how the pharmacovigilance system and quality system interact to achieve compliance. The risk-based approach to auditing the PV system and quality system The maintenance of 'inspection readiness' Explore how to investigate the complex PV system Discussions about how to monitor and maintain the PV system and assure compliance. Course Objectives Clarify what has to be done: Explore application of the legal requirements. Explore how to do what has to be done: Adopt a systemic approach to systematically investigate or implement and maintain the PV system and quality system Examine how a compliant PV system and a compliant quality system interact to achieve compliance with regulatory requirements for PV Explore how to investigate the complexity of the PV system. Discus how to identify what is missing or what needs to be improved: Discuss how to monitor and maintain the PV system and assure compliance. This course will assist delegates with: An understanding of key system principles, A practical approach to implementing, maintaining and monitoring the PV system and its quality system A procedure to share expertise to increase efficiency and confidence. This course is structured to encourage delegates to: Discuss and develop ideas - Share knowledge and experiences - Solve specific problems. By the end of the course delegates will be able to: Understand better the pharmacovigilance system, its quality system and how the components interact to achieve the objectives of pharmacovigilance Investigate, and analyse the pharmacovigilance system and to identify what is missing and what needs to be improved. Tutors Tutors will be comprised of (click the photos for biographies): Jana Hyankova Head of PV Department, IVIGEE Services a.s. Programme Please note timings may be subject to alteration. Day 1 08:30 Welcome, registration, course objectives and introduction to work groups Housekeeping notices, meet other delegates, explore how to work in your work group, course objectives. Clarify the definition and objectives of Pharmacovigilance. 09:30 The Regulatory Framework for Pharmacovigilance Identify the relevant regulations and directives. Explore GVP guidance, structure of the modules and standard format of each module. 10:00 The Pharmacovigilance System Exploration of how to organise what has to be done, communications. Exploration of the structures and processes for pharmacovigilance. 10:30 Break 11:00 Workshop 1 and Feedback Exploring an organisational model of the pharmaceutical company- cooperation between PV and other stakeholders. 12:00 The Quality System for pharmacovigilance Exploration of the structures, processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 13:00 Lunch 14:00 Workshop 2 and Feedback The quality system puzzle. Explore the organisation of the PV quality system and how it interacts with the PV system. 14:30 The Quality System for pharmacovigilance Exploration of the structures processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 15:00 Description of PV System 15:30 Break 15:30 Workshop 3 and Feedback The quality system puzzle Explore the organisation of the PV quality system and how it interacts with the PV system. 16:00 The Pharmacovigilance Safety Master File Construction of the Pharmacovigilance System Master File and its purpose. 17:00 Workshop 3 and Feedback Description of PV System. 18:00 End of Day Day 2 08:30 Drug Safety in the Clinical Trial Environment - Part 1 Information flow and responsibilities of the sponsor. 09:30 Workshop 4 and Feedback Drug Safety in the Clinical Trial environment: Information flow and responsibilities of the sponsor. 10:30 Break 11:00 Drug Safety in the Clinical Trial Environment - Part 2 Information flow and responsibilities of the sponsor. 12:00 Lunch 13:00 Workshop 5 and Feedback Drug Safety in the clinical trial environment: Information flow and responsibilities of the sponsor. 13:30 Processing of Safety Data Exploration of safety data processing, verification, validation, follow up, formatting and collation, reporting requirements, quality and data management. 15:00 Break 15:30 EudraVigilance Exploration of how EudraVigilance supports the PV system. 16:15 Signal Detection and Evaluation/Risk Benefit Assessment: Pharmacovigilance Risk Assessment Committee (PRAC): What is a signal? What are the regulatory requirements? How is signal detection and evaluation conducted? Qualitative and quantitative methods of signal detection. Risk benefit assessment. 17:00 Risk Management Plans A cornerstone of Pharmacovigilance safety communications, direct healthcare professional communication 18:00 End of Day Day 3 08:30 The Pharmacovigilance Risk Assessment Committee (PRAC) Exploration of how good practice is achieved. Composition, role and responsibilities. Examples of referrals. 09:15 Development Safety Update Reports (DSURs): Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:30 Break 11:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 12:00 Workshop 6 and Feedback To explore the compilation and submission of the PSUR. 13:00 Lunch 13:30 Role of the QPPV Exploration of the legal responsibilities of the QPPV and the MAH. 14:30 Break 15:00 Workshop 7 and Feedback To explore the challenges faced by the QPPV. 15:30 End of course Extra Information Face-to-Face Course Course material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device< Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD Points 23 Points Development Level Develop

Search By Location
- Regulations Courses in London
- Regulations Courses in Birmingham
- Regulations Courses in Glasgow
- Regulations Courses in Liverpool
- Regulations Courses in Bristol
- Regulations Courses in Manchester
- Regulations Courses in Sheffield
- Regulations Courses in Leeds
- Regulations Courses in Edinburgh
- Regulations Courses in Leicester
- Regulations Courses in Coventry
- Regulations Courses in Bradford
- Regulations Courses in Cardiff
- Regulations Courses in Belfast
- Regulations Courses in Nottingham