- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Report Writing 1 Day Training in Cardiff
By Mangates
Report Writing 1 Day Training in Cardiff

Report Writing 1 Day Training in Carlisle
By Mangates
Report Writing 1 Day Training in Carlisle

PRINCE2® Practitioner
By London School of Science and Technology
The PRINCE2® Practitioner course provides delegates with in-depth knowledge of project management methodologies. In this 2-day PRINCE2® Practitioner course enables learners to tailor the PRINCE2® methodology to any given project scenario. At the end of this PRINCE2® Practitioner course, delegates will be able to do delegating tolerances Course Overview The PRINCE2® Practitioner course provides delegates with in-depth knowledge of project management methodologies. In this 2-day PRINCE2® Practitioner course enables learners to tailor the PRINCE2® methodology to any given project scenario. They will learn about various essential topics such as communication management approach, tailoring the SU process, giving Ad Hoc direction, setting up the project controls, risk management procedure, PRINCE2® quality requirements, PRINCE2® approach to plan, tailoring SB, and many more. At the end of this PRINCE2® Practitioner course, delegates will be able to do delegating tolerances and report actual and forecast progress effectively. They will also be able to properly prepare the risk management, change control approach, quality management, and communication management approaches. The PRINCE2® Practitioner enables learners to apply their acquired knowledge and obtain highly reputed jobs with upgraded salaries. Concepts covered: • Balance of justification • Create the project plan • Quality audit trail • Quality review technique • Designing a plan • Gantt chart and tailoring • Change control approach Who should attend this PRINCE2® training course? This PRINCE2® Practitioner training course is for anybody interested in the field of project management. This PRINCE2® course is also intended for anyone looking to build their knowledge of how to tailor the PRINCE2® method to workplace scenarios. Other individuals that would benefit from undertaking PRINCE2® certifications include: • Project Managers • Aspiring Project Managers • Project Board Members • Project Support Staff • Office Support and Line Managers • Product Deliver Managers • Senior Responsible Owners • Change Analysts PRINCE2® Practitioner Prerequisites: It is required that delegates provide sufficient evidence of having satisfied the prerequisites before attending the PRINCE2® Practitioner training course. Delegates must hold the 2009 or 2017 version of the PRINCE2® Foundation certification or another valid qualification such as PMP, CAPM, or an IPMA Level A-D qualification. What’s Included in this PRINCE2® Practitioner Training Course? The following is included in this PRINCE2® Practitioner course: • PRINCE2® Practitioner Examination • Pre-course material • Post-course material • PDUs • Experienced PRINCE2® Instructor • Certificate • Refreshments Course Content: Module 1: Organisation Theme: • Four Levels of Management • PRINCE2® Organisation Requirements • Project Management Team • Project Management Team Roles • Project Board • Project Assurance • Change Authority • Project Support • Communication Management Approach Module 2: Starting Up a Project (SU): • Process Overview • Feasibility Study and Mandate • Appoint the Executive and the Project Manager • Capture Previous Lessons • Design and Appoint the Project Management Team • Prepare the Outline Business Case • Project Product Description • Select the Project Approach and Assemble the Project Brief • Plan the Initiation Stage • Tailoring the SU Process Module 3: Directing a Project (DP): • Authorise Initiation • Authorise the Project • Authorise a Stage or Exception Plan • Authorise Project Closure • Give Ad Hoc Direction • Tailoring the DP Process • Theme Overview • Balance of Justification • Continued Business Justification • PRINCE2® Requirements • Contents of a Business Case • Business Case Development • Benefits Management Approach • Key Responsibilities Module 4: Initiating a Project (IP): • Agree the Tailoring Requirements • Prepare the Risk Management Approach • Prepare the Change Control Approach • Prepare the Quality Management Approach • Prepare the Communication Management Approach • Set Up the Project Controls • Create the Project Plan • Prepare the Benefits Management Approach • Assemble the Project Initiation Documentation • Tailoring the IP process Module 5: Risk Theme: • Risk Definition • Effective Risk Management • PRINCE2® Risk Requirements • Risk Management Approach • Probability/Impact Grid • Risk Register • Risk Management Procedure • Identify Step • Risk Budget • Key Responsibilities Module 6: Quality Theme: • Quality Definitions • Quality Management • Quality Planning and Control • What is Quality Assurance? • PRINCE2® Quality Requirements • PRINCE2® Quality Documentation Requirements • Quality Management Approach • Quality Audit Trail • Project Product Description • Product Description • Quality Review Technique • Quality Review Roles/Responsibilities • Quality Review Meeting • Off-Specifications and Concessions • Review Follow-Up • Quality Review Benefits • Key Responsibilities • Communication Management Approach Module 7: Plans Theme: • Dealing with the Planning Horizon • PRINCE2® Planning Requirements • Documentation Requirements • Project and Stage Plans • Team Plans and Work Packages • Plans Relationship • What is in a Plan? • PRINCE2® Approach to Plans • Designing a Plan • Delivery Approaches • Defining and Analysing the Products • Product Breakdown Structures • Product Description • Product Flow Diagram • Identify the Activities and Dependencies • Preparing Estimates • Preparing a Schedule • Documenting the Plan • Analysing Risks to a Plan • Gantt Chart and Tailoring • Key Responsibilities Module 8: Progress Theme: • Progress Definition • PRINCE2® Requirements • Progress Control • Management by Exception • Delegating Tolerances and Reporting Actual and Forecast Progress • Types of Control • Management Products and Progress Control Module 9: Change Theme: • Issue Definition • PRINCE2® Approach to Change • PRINCE2® Change Documentation • Issue Register • Change Control Approach • Change Budget • Issue and Change Control Procedure • Issue Report • Exception Report Module 10: Controlling a Stage (CS): • Activity Breakdown • Authorise a Work Package • Work Package • Review Work Package Status • Receive Completed Work Packages • Review the Management Stage Status • Report Highlights • Highlight Report • Capture and Assess Issues and Risks • Escalate Issues and Risks • Take Corrective Action • Tailoring CS Module 11: Managing Product Delivery (MP): • Accept a Work Package • Execute a Work Package • Checkpoint Report • Deliver a Work Package • Tailoring MP Module 12: Managing a Stage Boundary (SB) : • Plan the Next Management Stage • What is in a Plan? • Update the Project Plan • Update the Business Case • Report the Management Stage End • End-Stage Report • Produce an Exception Plan • Tailoring SB Module 13: Closing a Project (CP): • Prepare Planned Closure • Hand Over Products • Evaluate the Project • End Project Report • Recommend Project Closure • Tailoring CP DURATION 2 Days WHATS INCLUDED Course Material Case Study Experienced Lecturer Refreshments Certificate

Organisation Programme - Bribery and corruption risk assessment
By Global Risk Alliance Ltd
Our training programme will provide those involved at any stage of the process for procuring goods and/or services within their organisations with the knowledge and skillset to identify and mitigate the threat posed by the breadth and multi-layered complexity of procurement fraud, corruption and associated financial crime and money laundering.

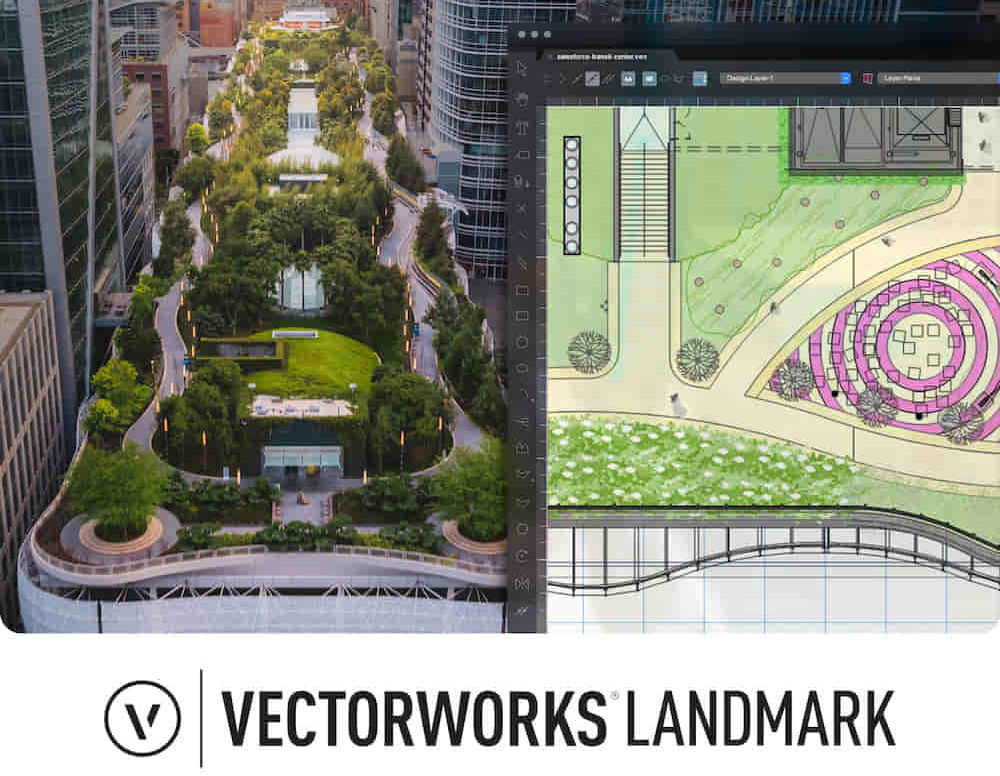
Landmark Training Course With Vectorworks
By ATL Autocad Training London
Who is this course for? Landmark Training Course With Vectorworks. Dive into terrain modeling, planting, irrigation, and site analysis guided by certified tutors. Master these tools for precise landscape designs and effective documentation. Check our Website Enrollment : 1-on-1 Landmark Training. Tailor your schedule. Mon to Sat between 9 am and 7 pm Call 02077202581 to book your slot. Duration: 16 hours. "Split these hours over multiple days as needed for your ideal schedule." Approach: In-person or live online. Landmark Training Course with Vectorworks: Basic to Intermediate Level Course Duration: 16 Hours Embark on a transformative journey with our Landmark Training Course tailored for landscape architects and designers. Over 16 intensive hours, dive deep into Vectorworks Landmark, mastering fundamental and intermediate techniques crucial for comprehensive 2D and 3D landscape design. Craft intricate site analyses, plans, irrigation systems, and elevate your designs with mesmerizing 3D visualizations. Explore custom plant symbols, detailed planting plans, and learn the art of efficient collaboration and customization. Course Highlights: I. Introduction to Vectorworks Landmark (1 hour) Explore Vectorworks Landmark for landscape design Master interface, tool usage, and project management II. Site Analysis and Site Plans (3 hours) Craft detailed site analyses and hardscape designs Work with contours, elevations, and terrain models III. Planting Plans (5 hours) Utilize the plant database for region-specific plant selection Create personalized plant symbols and comprehensive planting plans IV. Irrigation Design (2 hours) Design and edit efficient irrigation systems and zones Integrate irrigation components into site plans V. 3D Visualization (3 hours) Create captivating 3D models with realistic textures and materials Enhance designs with advanced lighting and special effects VI. Customization (1 hour) Tailor the interface for efficient landscape design Create custom object styles and resource libraries VII. Collaboration and Sharing (1 hour) Seamlessly import/export data from other platforms Share designs effectively with colleagues and collaborators VIII. Conclusion and Next Steps (1 hour) Review course content comprehensively Receive guidance on further learning resources Engage in a Q&A session and provide valuable feedback Enhance your landscape design expertise and unleash your creativity. Enroll now in our Vectorworks Landmark Basic to Intermediate Training and transform your designs. Download Vectorworks Landmark By the end of the Vectorworks Landmark Training Course, participants will: Understand the key features and functionalities of Vectorworks Landmark for landscape design. Demonstrate proficiency in using essential tools for site analysis, site plans, and hardscape design. Create detailed planting plans, selecting appropriate plants, and understanding their compatibility and growth patterns. Design efficient and effective irrigation systems, including generating irrigation reports. Create 3D visualizations of landscape designs, applying textures, materials, lighting, and special effects. Customize the interface and create personalized object styles and resources. Collaborate and share landscape design drawings with other software users. Have the foundation to pursue further learning and exploration in landscape design using Vectorworks Landmark. Mastering Foundations Begin your project by organizing your files and importing survey data. Learn to sketch existing buildings using Building Shell tools and model neighboring structures with Massing Model. Explore different methods for laying out survey points and marking existing features with precision. Objectives: Review File Organization Techniques Import Survey Data (DWG Import) Create Building Structures with Building Shell Tools Utilize Triangulation and Arc Tool for Precision Master Various Tape Measurement Techniques Elevating Your Design Enhance your survey with detailed ground, existing trees, and fences. Conduct shadow analysis to optimize planting locations. Dive into the Vectorworks Plant tool, your key design companion. Objectives: Develop Detailed Ground Surfaces Incorporate Existing Trees and Fences Design with Railing Fence Tool Conduct Shadow Analysis using Heliodon Tool Utilize Plant Tool Modes for Plant Placement Access Existing Plant Libraries and Customize Plants in 2D/3D Crafting Landscapes Create vibrant plant mixes using Landscape Area tool and apply them across your site models. Design intricate hardscapes, aligning them effortlessly even in complex paving scenarios. Learn to use components for detailed reporting, cut and fill calculations, and precise detailing. Explore custom object creation and site furniture placement. Objectives: Design Landscape Areas and Define Custom Plant Mixes Create Hardscapes and Define Custom Paving Constructions Generate Reports and Tags for Landscape Areas and Hardscapes Access and Manage Objects in Resource Manager Craft Custom Objects and Site Furniture Polished Presentation Present your designs professionally using Sheet layers and viewports. Create Section viewports to cut through your model and Detail viewports to focus on specific areas. Enhance visual appeal with mood boards and annotations, ensuring a refined, detailed presentation. Objectives: Craft Sheet Layers for Presentation Create Plan, Elevation, and Perspective Viewports Generate Section and Detail Viewports Annotate Viewports for Clear Communication Incorporate Images and Plant Reports for Comprehensive Presentations Master Vectorworks Landmark: Gain expertise in essential and advanced 2D/3D landscape design tools for precision and efficiency. Boost Efficiency: Learn time-saving techniques and workflows tailored to Vectorworks Landmark, enhancing productivity. Versatile Landscape Skills: Develop proficiency in site analysis, planting plans, hardscapes, and irrigation systems for diverse projects. Industry-Ready Expertise: Acquire sought-after skills in landscape architecture, paving the way for career growth. Flexible Learning: Access recorded lessons for convenient review and receive lifetime email support for ongoing guidance.
Finance for the non-accountant (In-House)
By The In House Training Company
No-one in business will succeed if they are not financially literate - and no business will succeed without financially-literate people. This is the ideal programme for managers and others who don't have a financial qualification or background but who nonetheless need a greater understanding of the financial management disciplines essential to your organisation. This course will give the participants a sound understanding of financial reports, measures and techniques to make them even more effective in their roles. It will enable participants to: Overcome the barrier of the accountants' strange language Deal confidently with financial colleagues Improve their understanding of your organisation's finance function Radically improve their planning and budgeting skills Be much more aware of the impact of their decisions on the profitability of your organisation Enhance their role in the organisation Boost their confidence and career development 1 Review of the principal financial statements What each statement containsOutlineDetail Not just what the statements contain but what they mean Balance sheets and P&L accounts (income statements) Cash flow statements Detailed terminology and interpretation Types of fixed asset - tangible, etc. Working capital, equity, gearing 2 The 'rules' - Accounting Standards, concepts and conventions Fundamental or 'bedrock' accounting concepts Detailed accounting concepts and conventions What depreciation means The importance of stock, inventory and work in progress values Accounting policies that most affect reporting and results The importance of accounting standards and IFRS 3 Where the figures come from Accounting records Assets / liabilities, Income / expenditure General / nominal ledgers Need for internal controls 'Sarbox' and related issues 4 Managing the budget process Have clear objectives, remit, responsibilities and time schedule The business plan Links with corporate strategy The budget cycle Links with company culture Budgeting methods'New' budgetingZero-based budgets Reviewing budgets Responding to the figures The need for appropriate accounting and reporting systems 5 What are costs? How to account for them Cost definitions Full / absorption costing Overheads - overhead allocation or absorption Activity based costing Marginal costing / break-even - use in planning 6 Who does what? A review of what different types of accountant do Financial accounting Management accounting Treasury function Activities and terms 7 How the statements can be interpreted What published accounts contain Analytical review (ratio analysis) Return on capital employed, margins and profitability Making assets work - asset turnover Fixed assets, debtor, stock turnover Responding to figures EBIT, EBITEDIA, eps and other analysts' measure 8 Other key issues Creative accounting Accounting for groups Intangible assets - brand names Company valuations Fixed assets / leased assets / off-balance sheet finance

A System Approach to Good Pharmacovigilance Practice
By Research Quality Association
Course Information In today's evolving landscape, pharmacovigilance (PV) systems face ongoing challenges due to global, national, and company-specific events. This course focuses on developing personnel equipped to navigate these complexities and improve the safe use of medicinal products. We emphasise continuous global thinking, communication, and strategic planning, ensuring adaptability across various levels. The course explores maintaining PV system functionality while adhering to regulatory requirements. Participants will apply European regulatory standards to enhance and sustain PV system effectiveness, contributing to improvement initiatives and ensuring operational integrity. The course covers PV system intricacies, regulatory compliance, and a 'systems approach' for auditors, quality assurance personnel, and PV practitioners. Through presentations and workshops, attendees gain insights into implementing and maintaining an effective PV system. Who should attend? Auditors Pharmacovigilance Quality System Managers Pharmacovigilance scientists The QPPV. Course benefits Throughout the course delegates will explore application of the legal requirements for the PV system and quality system and how to assure these systems. They will demonstrate their ability to contribute to: A systematic investigation of the pharmacovigilance system and its quality system Examination of how the pharmacovigilance system and quality system interact to achieve compliance. The risk-based approach to auditing the PV system and quality system The maintenance of 'inspection readiness' Explore how to investigate the complex PV system Discussions about how to monitor and maintain the PV system and assure compliance. Course Objectives Clarify what has to be done: Explore application of the legal requirements. Explore how to do what has to be done: Adopt a systemic approach to systematically investigate or implement and maintain the PV system and quality system Examine how a compliant PV system and a compliant quality system interact to achieve compliance with regulatory requirements for PV Explore how to investigate the complexity of the PV system. Discus how to identify what is missing or what needs to be improved: Discuss how to monitor and maintain the PV system and assure compliance. This course will assist delegates with: An understanding of key system principles, A practical approach to implementing, maintaining and monitoring the PV system and its quality system A procedure to share expertise to increase efficiency and confidence. This course is structured to encourage delegates to: Discuss and develop ideas - Share knowledge and experiences - Solve specific problems. By the end of the course delegates will be able to: Understand better the pharmacovigilance system, its quality system and how the components interact to achieve the objectives of pharmacovigilance Investigate, and analyse the pharmacovigilance system and to identify what is missing and what needs to be improved. Tutors Tutors will be comprised of (click the photos for biographies): Jana Hyankova Head of PV Department, IVIGEE Services a.s. Programme Please note timings may be subject to alteration. Day 1 08:30 Welcome, registration, course objectives and introduction to work groups Housekeeping notices, meet other delegates, explore how to work in your work group, course objectives. Clarify the definition and objectives of Pharmacovigilance. 09:30 The Regulatory Framework for Pharmacovigilance Identify the relevant regulations and directives. Explore GVP guidance, structure of the modules and standard format of each module. 10:00 The Pharmacovigilance System Exploration of how to organise what has to be done, communications. Exploration of the structures and processes for pharmacovigilance. 10:30 Break 11:00 Workshop 1 and Feedback Exploring an organisational model of the pharmaceutical company- cooperation between PV and other stakeholders. 12:00 The Quality System for pharmacovigilance Exploration of the structures, processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 13:00 Lunch 14:00 Workshop 2 and Feedback The quality system puzzle. Explore the organisation of the PV quality system and how it interacts with the PV system. 14:30 The Quality System for pharmacovigilance Exploration of the structures processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 15:00 Description of PV System 15:30 Break 15:30 Workshop 3 and Feedback The quality system puzzle Explore the organisation of the PV quality system and how it interacts with the PV system. 16:00 The Pharmacovigilance Safety Master File Construction of the Pharmacovigilance System Master File and its purpose. 17:00 Workshop 3 and Feedback Description of PV System. 18:00 End of Day Day 2 08:30 Drug Safety in the Clinical Trial Environment - Part 1 Information flow and responsibilities of the sponsor. 09:30 Workshop 4 and Feedback Drug Safety in the Clinical Trial environment: Information flow and responsibilities of the sponsor. 10:30 Break 11:00 Drug Safety in the Clinical Trial Environment - Part 2 Information flow and responsibilities of the sponsor. 12:00 Lunch 13:00 Workshop 5 and Feedback Drug Safety in the clinical trial environment: Information flow and responsibilities of the sponsor. 13:30 Processing of Safety Data Exploration of safety data processing, verification, validation, follow up, formatting and collation, reporting requirements, quality and data management. 15:00 Break 15:30 EudraVigilance Exploration of how EudraVigilance supports the PV system. 16:15 Signal Detection and Evaluation/Risk Benefit Assessment: Pharmacovigilance Risk Assessment Committee (PRAC): What is a signal? What are the regulatory requirements? How is signal detection and evaluation conducted? Qualitative and quantitative methods of signal detection. Risk benefit assessment. 17:00 Risk Management Plans A cornerstone of Pharmacovigilance safety communications, direct healthcare professional communication 18:00 End of Day Day 3 08:30 The Pharmacovigilance Risk Assessment Committee (PRAC) Exploration of how good practice is achieved. Composition, role and responsibilities. Examples of referrals. 09:15 Development Safety Update Reports (DSURs): Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:30 Break 11:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 12:00 Workshop 6 and Feedback To explore the compilation and submission of the PSUR. 13:00 Lunch 13:30 Role of the QPPV Exploration of the legal responsibilities of the QPPV and the MAH. 14:30 Break 15:00 Workshop 7 and Feedback To explore the challenges faced by the QPPV. 15:30 End of course Extra Information Face-to-Face Course Course material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device< Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD Points 23 Points Development Level Develop

Mini Medics First Aid
By Aplus Medical Services & Training
Introduction to First Aid Age Range: 8yrs – 11yrs Course Length: 90 Minutes Cost: £20 + vat per child Maximum of 12 SYLLABUS A range of subjects are covered, including:- Responsibilities & Reporting Assessment of the situation Dealing with an unresponsive casualty Basic Hygiene in First-Aid CERTIFICATION Resuscitation & AED Awareness Anatomy Minor injuries Bleeding control Burns Choking Epilepsy Shock Maximum of 12 People All learners will have the skills and knowledge to provide the organisation with Emergency First Aiders who can provide treatment to their casualties in a prompt, safe and effective manner. This accredited One-Day Qualification meets the requirements of the Health & Safety (First Aid) Regulations 1981 and the Health and Safety (First Aid) Regulations (Northern Ireland) 1982. The Certificate is valid for 3 years and will expire from the date on the certificate. Children’s First Aid • Mini Medics First Aid & Mental Health Training Courses for Children At APLUS Medical Services & Training, we believe everyone should have access to quality Medical Training. Whether you’re a parent, a teacher, a student or simply someone who wants to learn Life-Saving Skills, we have the right course for you. Our Children’s First Aid training courses are designed with the unique needs of children in mind. We understand that children are not just small adults – they have specific medical needs and emergencies that require specialised knowledge and skills. Our Children’s First Aid courses cover a wide range of topics, including CPR for Infants and Children, Choking Prevention and Intervention, Wound Care, and recognising signs of common childhood illnesses and conditions. Our experienced Instructors provide hands-on training during the Children’s First Aid Courses using child-sized mannequins and other equipment specifically designed for Paediatric First-Aid training. This ensures that our trainees are well-prepared to handle real-life emergencies involving children.
Telephone Training - Gamma Horizon
By Telephone Trainers Ltd
Polycom Handsets and Voicemail Soft Client Horizon Collaborate Desktop and Mobile App Receptionist Console Integrator Agent Client Supervisor Client Akixi Reporting Horizon Contact Agent Horizon Contact Supervisor Horizon Contact Admin User Web Portal User Admin Portal

COSHH & RIDDOR
By Prima Cura Training
This course aims to provide a more in depth understanding of COSHH & RIDDOR.

Search By Location
- Reporting Courses in London
- Reporting Courses in Birmingham
- Reporting Courses in Glasgow
- Reporting Courses in Liverpool
- Reporting Courses in Bristol
- Reporting Courses in Manchester
- Reporting Courses in Sheffield
- Reporting Courses in Leeds
- Reporting Courses in Edinburgh
- Reporting Courses in Leicester
- Reporting Courses in Coventry
- Reporting Courses in Bradford
- Reporting Courses in Cardiff
- Reporting Courses in Belfast
- Reporting Courses in Nottingham