- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
The Mind-Body Connection: Emotion, Movement and Calm
By Human Givens College
The latest mind body research gives us profound insights into the effects on mental health of 'physical' factors, including breathwork and time in Nature, new ways to improve mental and physical health, and more… This wide-ranging day will deepen your knowledge of the mind-body system and the impact ‘physical factors’ can have on our emotions, state of mind and memory – with additional ways to help people quickly and effectively… Accredited CPD: 6 hours Length: 1 day (9.30am - 4.00pm) A brilliant framework to understand the neurobiology of our needs and how to manage stressDR ALLY JAFFEE, NHS DOCTOR Why take this course Having a deeper understanding of the latest research into the effects on mental health of ‘physical’ factors – such as movement and exercise, time in Nature, ‘breathwork’ and real-time connection with others – enables us to tailor powerful therapeutic interventions and deliver more informed psycho-education. This jam-packed day also covers the physiology of responses such as ‘freeze, fight or flight’, the effects of physical activity on emotional, mental, memory and brain health, why stress can become chronic, the many ways we can activate our innate capacity to restore calm through our breath and attention, why these relaxation techniques work and what they do for us physically. The findings you will explore on the workshop have implications for many areas of mental health, even addiction, and give us additional ways to help people resolve many difficulties quickly and effectively, as well as improve overall health. The HG approach recognises that meeting our emotional needs and using our resources well are key to good mental health – yet the impact of ‘physical’ factors on our emotions and state of mind can be just as profound. Even though our physical needs may appear obvious – a healthy diet, regular physical exercise etc – we know that most of the chronic diseases sweeping through the Western World are linked to “lifestyle”, indicating that many people are not actually meeting their physical needs in healthy balanced ways – despite the vast array of information available to them. Finding clarity about how best to meet our own physical needs can also help us maintain our capacity to help others. Throughout the day, Dr Andrew Morrice, a practising GP, draws on both the latest research and his decades of experience managing the complex combination of mental and physical health problems in General Practice (20 of those years using the Human Givens model). We will sift the sense from the nonsense, and see the fundamentals behind the buzzwords and trends, making it easier to think clearly about our own health as well as that of our patients or clients… Really insightful course with an amazing way into practical applications of the HG concepts...ALIYA DRAKE, HG THERAPIST What will you learn How the now well-known human ‘needs and resources’ relate to our evolutionary past, and physical lives The many ways physical movement affects the functioning of the mind and our emotions A deeper look into the physiology of emotional responses, such as ‘fight, flight, freeze’ and their impact on health Greater knowledge of the fundamentals of how we can find calm – and the very many ways these can be used to help people New information from the latest mind/body research that has implications for therapeutic lifestyle changes Also covered: What is ‘health’? And how do we get it? Key practices to support your own health, as well as your clients’ How to avoid the unhelpful psychology and aversive ‘pattern match’ that many of us have when it comes to ‘exercise’ Whether we can really be addicted to exercise and if so why? A deeper understanding about the role of “Green Space” in mental health Increased knowledge of the ways in which physical changes in the body contribute to emotional distress The many ways in which movement and physical activity are connected with mental health How thinking and moving, memory and place are inextricably linked Why stress becomes chronic (long-lasting) Greater clarity about the role some emotions play in creating physical ill health – and the powerful ways in which other emotional states promote health (including the key role of oxytocin) The science of the relaxation and oxytocin responses, and how these relate to many types of addiction, including smoking Organising ideas to understand how 6 different types of relaxation practice relate to each other A practical exploration of the many ways the relaxation response can be activated through breath and attention – including a new technique for dealing with panic Why some people may have come to believe that ‘the breathing’ can’t or won’t help them Summaries of the role of diet and sleep on our health – along with the factors considered today in ‘Therapeutic Lifestyle Change’ Time to ask our expert tutor questions and benefit from group discussions Course Programme The ‘The Mind-Body Connection in depth: Movement, Emotion and Calm’ course starts at 9.30am and runs until 4.00pm. From 8.30am Registration (Tea and coffee served until 9.25am) 9.30am What is health and how do we 'get it'? 10.45am Discussion over tea/coffee 11.15am The physiology of fear and calm 12.45pm Lunch (included) 1.30pm The physiology of rapport (and other topics) 2.45pm Discussion over tea/coffee 3.00pm Physical domains of mental health 4.00pm Day ends Who is this course suitable for? This course is open to anyone interested in mental and/or physical health If you enjoyed the original 1-day Mind-Body Connection course and would like time to explore in more detail the themes covered in the first half of that course, this new course is for you Please note: you don’t need to have attended the previous Mind-Body Connection course to come on this one, or its sister course ‘Food, Mood and Sleep’. This course has been independently accredited by the internationally recognised CPD Standards Office for 6 hours of CPD training. On completion of this training you’ll receive CPD certificates from the College and the CPD Standards Office.

Computing - GCSE Syllabus - In Person Tuition, London, Lambeth, Wandsworth, Merton, Southwark, Kensington & Chelsea
5.0(8)By GLA Tutors Home or Online
Computer Science GCSE Syllabus The GCSE Computer Science Tutor Syllabus is designed to provide tutors in England with a comprehensive framework for teaching the GCSE Computer Science curriculum effectively. This syllabus aims to equip tutors with the necessary knowledge and skills to support students in their understanding and application of core computer science concepts. Module 1: Introduction to Computer Science - Overview of computer science and its relevance in today's world - Understanding the components of a computer system - Introduction to algorithms and problem-solving techniques - Exploration of programming languages and their uses Module 2: Computer Hardware - Understanding the main components of a computer system, including CPU, memory, and storage devices - Exploring input and output devices and their functionalities - Understanding the role of operating systems and software in computer systems Module 3: Software Development - Introduction to programming concepts and languages (e.g., Python or Java) - Understanding variables, data types, and operators - Building algorithms and logical reasoning skills - Introduction to flowcharts and pseudocode - Implementation of simple programs and debugging techniques Module 4: Data Representation - Understanding binary, hexadecimal, and denary number systems - Representation of text, images, and sound using binary - Introduction to data compression and encryption techniques Module 5: Computer Networks - Understanding the basics of computer networks, including LAN, WAN, and the Internet - Introduction to network topologies, protocols, and security - Exploring the impact of digital communication on society Module 6: Cybersecurity and Ethical Issues - Understanding the importance of cybersecurity and data protection - Introduction to common threats and vulnerabilities - Exploring ethical issues related to computer science, such as privacy and intellectual property rights Module 7: Algorithms and Programming Techniques - Advanced programming concepts, including conditionals, loops, and functions - Introduction to sorting and searching algorithms - Exploring data structures, such as arrays and lists Module 8: System Architecture - Understanding the structure and function of a CPU - Introduction to memory hierarchy and cache - Exploring the Von Neumann architecture and its limitations Module 9: Computational Thinking and Problem Solving - Advanced problem-solving techniques using computational thinking - Introduction to algorithms for complex problems - Exploring algorithmic efficiency and optimization techniques Module 10: Exam Preparation and Revision - Reviewing key concepts covered throughout the syllabus - Practicing past exam questions and providing guidance on exam techniques - Supporting students with exam preparation strategies Please note that the duration and depth of each module can vary depending on the level of expertise required and the specific needs of the learners. Additionally, it's important to adapt the curriculum to the learners' proficiency levels, whether they are A Level/GCSE students or adult learners with different experience levels.

Game Design Training: 3ds Max and Unity 3D Personalized
By Real Animation Works
Game design training face to face training customised and bespoke.

Vectorworks and Sketchup Help in Interior Design projects
By Real Animation Works
Vectorworks Course face to face One to one
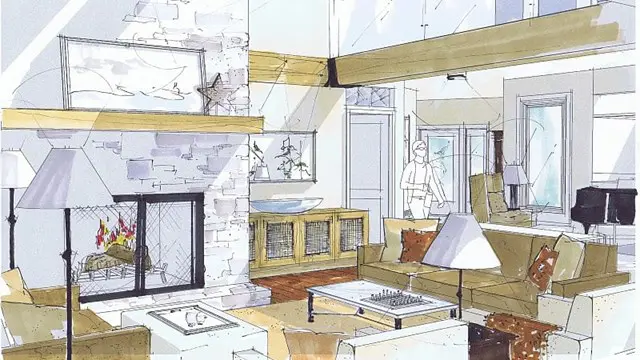
FURNITURE DESIGN TRAINING
By Real Animation Works
Furniture design face to face training customised and bespoke.
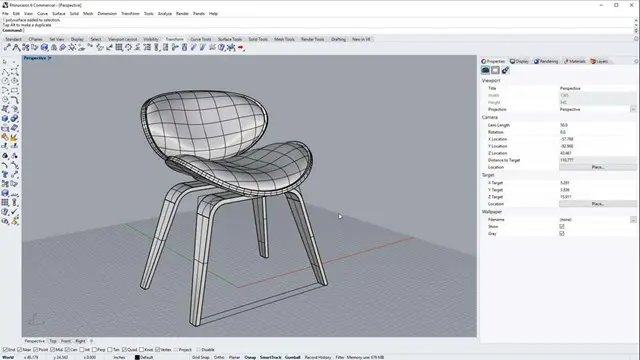
Training Course for Architects & Interior Exterior Designers
By Real Animation Works
1-2-1 face to face training customised and bespoke.

Become A Highly Qualified Aesthetic Laser Therapist
By Cosmetic College
Comprehensive Training for Aspiring Laser Aesthetic Technicians Our laser courses are meticulously designed to offer a complete understanding of laser procedures. We guide you through three levels of training - foundation, intermediate, and advanced - ensuring you are ready to start treating clients in just a few weeks. With the VTCT NVQ Level 4 Certificate in Laser, IPL (Intense Pulse Light) and Skin Rejuvenation, you'll be able to provide high-quality, safe, and effective laser hair removal treatments. This advanced qualification is perfect for beauty therapists and medical specialists who are passionate about aesthetics and want to expand their skillset. Hands-On Laser Hair Removal Training During your training, you'll learn to conduct thorough consultations, analyze hair and skin types, identify Fitzpatrick skin types, determine the number of sessions required, set machine parameters, perform patch tests, conduct full treatments, and provide aftercare advice. We deliver our training through a blend of online learning modules and in-person practical sessions. You'll be able to witness professional live treatment demonstrations and gain hands-on experience under the guidance of our expert tutors. The Laser Hair Removal Course is meticulously tailored for practitioners aspiring to expand their service offerings, or for those seeking to enhance their skills within larger clinics or hospital settings. The list includes aestheticians, beauty therapists, healthcare professions such as doctors, dentists, dental nurses and nurse prescribers.
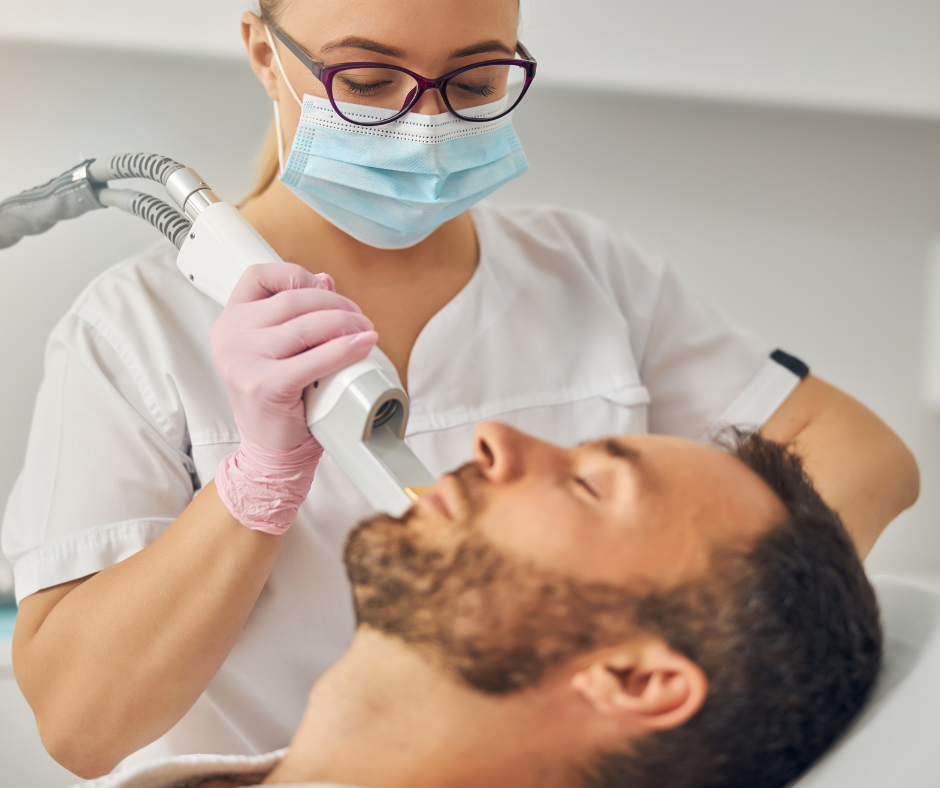
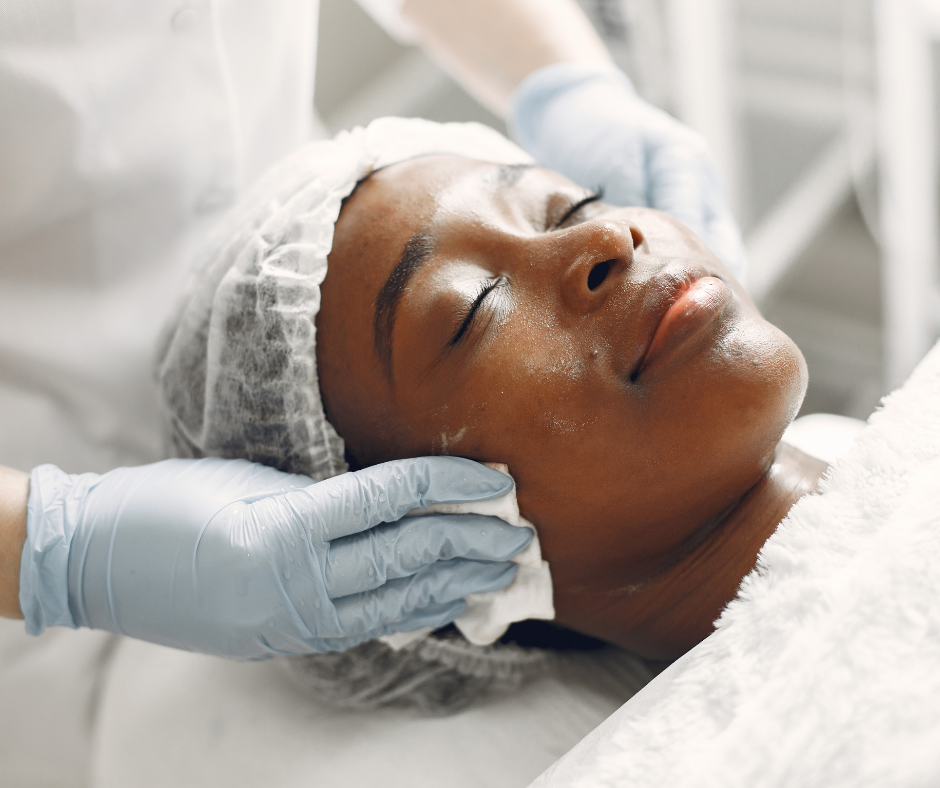
VTCT Level 2 Facial and Skincare Course
By Cosmetic College
Welcome to the Cosmetic College, where we are dedicated to providing high-quality VTCT NVQ beauty courses in London. Our VTCT Level 2 Facial and Skin Care Course is designed to help you develop the skills and knowledge you need to succeed in the beauty industry. We understand that time is of the essence, which is why we offer a comprehensive course that covers everything from cleansing and toning to exfoliating and moisturising. With our expert guidance and hands-on training, you'll learn how to tailor your treatments to meet the unique needs of each client. We also understand the importance of patience when it comes to mastering the art of beauty therapy. Our experienced tutors will take the time to guide you through the course, answering any questions you may have and providing feedback to help you improve your technique. We believe in setting a solid foundation for your future success, and that means taking the time to ensure you're fully equipped to provide exceptional beauty treatments. Upon completion of the course, you'll receive a VTCT Level 2 Diploma in Facial and Skin Care, which is widely recognised by employers across the UK. This qualification will open up a variety of career opportunities, from working in salons and spas to starting your own beauty business. We know that everyone has different schedules and commitments, which is why we offer flexible learning options. Whether you're looking for part-time or full-time courses, we'll work with you to create a schedule that fits your needs. Join us today and start your journey to becoming a successful beauty therapist. Contact us to learn more about our VTCT NVQ beauty courses in London. We look forward to helping you achieve your career goals!
Search By Location
- tutor Courses in London
- tutor Courses in Birmingham
- tutor Courses in Glasgow
- tutor Courses in Liverpool
- tutor Courses in Bristol
- tutor Courses in Manchester
- tutor Courses in Sheffield
- tutor Courses in Leeds
- tutor Courses in Edinburgh
- tutor Courses in Leicester
- tutor Courses in Coventry
- tutor Courses in Bradford
- tutor Courses in Cardiff
- tutor Courses in Belfast
- tutor Courses in Nottingham

