- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
12215 Courses
HACCP Trainer - On site Level 3 HACCP Training - Nationwide
By Kitchen Tonic Training Company and Food Safety Consultants
HACCP Trainer. on site delivery nationwide

Let’s Talk About Trauma
By Empowerful Living
A free, interactive session for women, about trauma and how to heal from it.

Hopeful Spouse Counseling That Save My Marriage http://dradodalovetemple.com
By Adoda love spell
LOVE SPELL

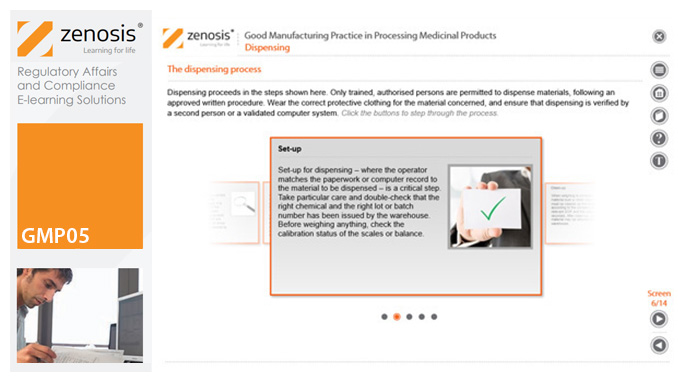
GMP05: Good Manufacturing Practice in Processing Medicinal Products
By Zenosis
Operations in the dispensary and on processing lines are at the heart of medicinal product manufacturing. This module describes how to carry out such operations in compliance with the requirements of Good Manufacturing Practice.
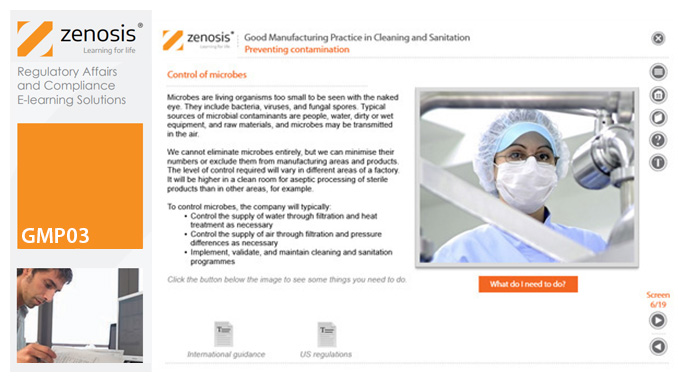
GMP03: Good Manufacturing Practice in Cleaning and Sanitation
By Zenosis
Cleaning and sanitation of premises and equipment are essential to efforts to prevent contamination of product, and they need to be done in compliance with Good Manufacturing Practice (GMP) regulatory requirements. This module shows why it is so important to do a good job, what to consider before and during each job, and how best to go about the work.
GMP06: Good Manufacturing Practice in Packaging Medicinal Products
By Zenosis
Packaging for medicinal products is subject to Good Manufacturing Practice rules similar to those for the products themselves. In this module we describe the functions that packaging must fulfil and the quality controls that are applied to packaging materials and operations. We set out the requirements for control of printed materials. We describe preparation, in-process control, and completion of a packaging run. Finally, we explain how to carry out reconciliation of packaging materials.

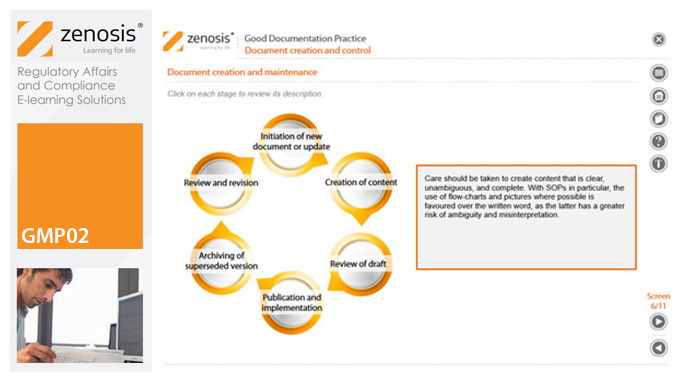
GMP02: Good Documentation Practice
By Zenosis
Good Manufacturing Practice (GMP) for medicinal products relies on documentation. Good Documentation Practice (GDocP) is that part of GMP that applies to the creation, maintenance, use, and retention of documents to provide assurance of the quality of products.
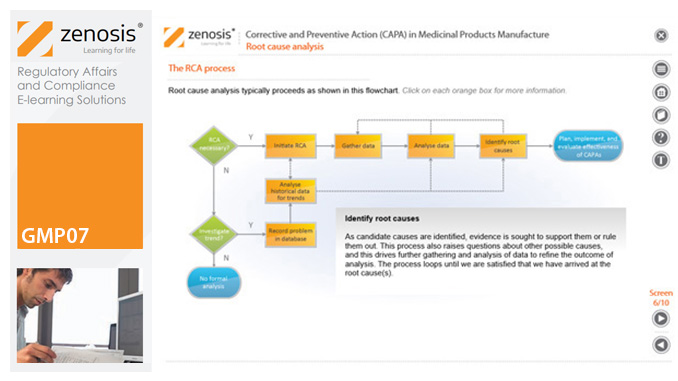
GMP07: Corrective and Preventive Action (CAPA) in Medicinal Products Manufacture
By Zenosis
A company’s Corrective and Preventive Action (CAPA ) system establishes how personnel should deal with manufacturing problems that have occurred or that may occur if not prevented. This module explains the principles of corrective and preventive action and describes typical CAPA procedure. It goes on to introduce root cause analysis and outline the role of progress tracking, escalating, and trending of CAPA procedures.
ESS02: Essentials of Monoclonal Antibodies
By Zenosis
This module will introduce you to monoclonal antibodies, explaining how they work, how they are made, and the many uses to which they are put.

DESIGNATED SAFEGUARDING LEAD FOR SCHOOLS & COLLEGES IN HOUSE TRAINING
By Child Protection Training Uk
This In House Training for the designated safeguarding lead in your school or college, we can offer a 4 or 6 hour course for 1 - 50 people, this can be delivered in your organisation during the day or evening at a times to suit your needs. Carry out your statutory responsibilities as a Designated safeguarding lead (DSL) with the DSL training courses and protect the children and young people you work with in schools and colleges in England.

Search By Location
- Safety Courses in London
- Safety Courses in Birmingham
- Safety Courses in Glasgow
- Safety Courses in Liverpool
- Safety Courses in Bristol
- Safety Courses in Manchester
- Safety Courses in Sheffield
- Safety Courses in Leeds
- Safety Courses in Edinburgh
- Safety Courses in Leicester
- Safety Courses in Coventry
- Safety Courses in Bradford
- Safety Courses in Cardiff
- Safety Courses in Belfast
- Safety Courses in Nottingham